추천 제품
분석
≥95%
형태
powder or crystals
특징
generation 3
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
>300 °C
작용기
phosphine
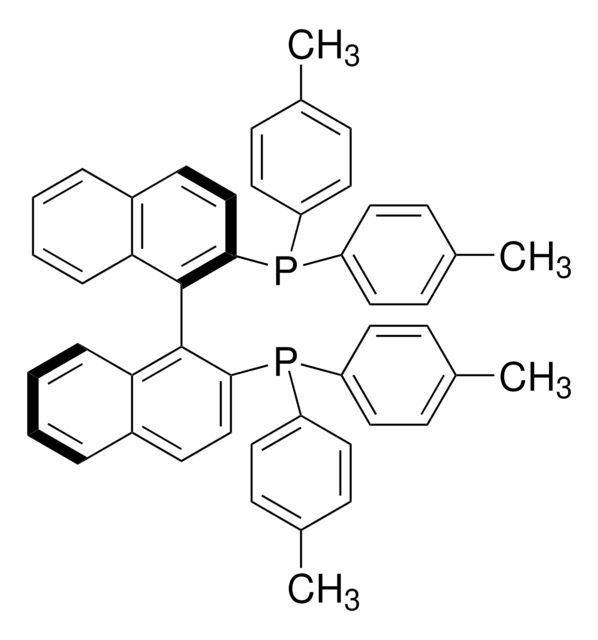
SMILES string
PC1=CC=C2C(C=CC=C2)=C1C3=C(C)C=CC4=C3C=CC=C4.NC(C=CC=C5)=C5C6=C([Pd]OS(C)(=O)=O)C=CC=C6.[Tol2].[Tol2].P
일반 설명
(R)-Tol-BINAP Pd G3 is a third generation (G3) Buchwald precatalyst. It is air, moisture and thermally stable and is highly soluble in a wide range of common organic solvents. It has long life in solutions. Qphos Pd G3 is an excellent reagent for palladium catalyzed cross-coupling reactions. Some of its unique features include lower catalyst loadings, shorter reaction time, efficient formation of the active catalytic species and accurate control of ligand: palladium ratio.
애플리케이션
(R)-Tol-BINAP Pd G3 can be used in the stereoselective synthesis of perfluoroalkyl-substituted enones by reacting four components, alkynes, iodoperfluoroalkanes, (hetero)arylboronic acids, and carbon monoxide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Sylwester Domański et al.
The Journal of organic chemistry, 82(15), 7998-8007 (2017-07-06)
A four-component Pd-catalyzed protocol for direct synthesis of perfluoroalkyl-substituted enones is reported. Under mild conditions and low catalyst loading, alkynes, iodoperfluoroalkanes, (hetero)arylboronic acids, and carbon monoxide are assembled into highly elaborate products with good yields and excellent regio- and stereoselectivities.
Pd-Catalyzed Carbonylative Carboperfluoroalkylation of Alkynes. Through-Space 13C-19F Coupling as a Probe for Configuration Assignment of Fluoroalkyl-Substituted Olefins.
Domanski S, et al.
The Journal of Organic Chemistry, 82(15), 7998-8007 (2017)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.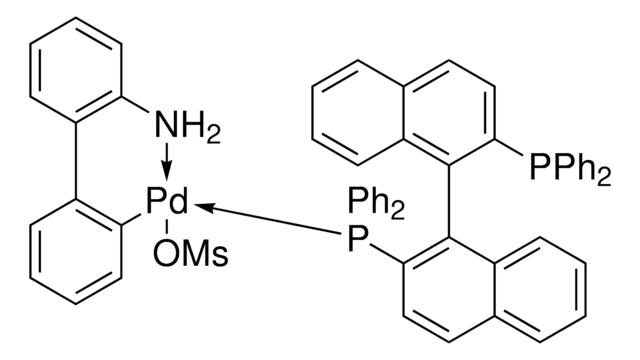
![Dichloro[2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]palladium(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/351/904/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3/640/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3.png)