768375
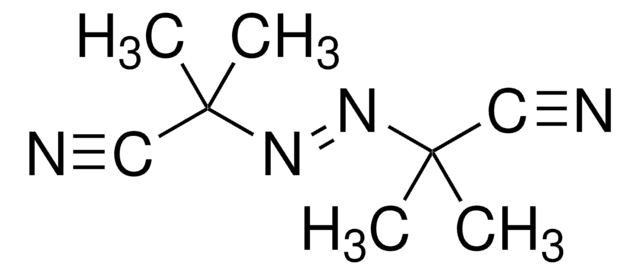
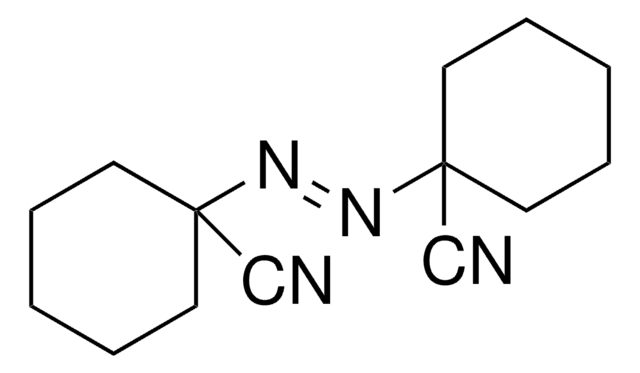
Azobisisobutyronitrile
12 wt. % in acetone
동의어(들):
2,2′-Azobis(2-methylpropionitrile) solution, radical initiator, α,α,′-Azoisobutyronitrile solution, AIBN solution
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C8H12N4
CAS Number:
Molecular Weight:
164.21
MDL number:
UNSPSC 코드:
12162002
PubChem Substance ID:
추천 제품
양식
liquid
Quality Level
농도
12 wt. % in acetone
refractive index
n20/D 1.368
density
0.808 g/mL at 25 °C
저장 온도
2-8°C
SMILES string
CC(C)(\N=N\C(C)(C)C#N)C#N
InChI
1S/C8H12N4/c1-7(2,5-9)11-12-8(3,4)6-10/h1-4H3/b12-11+
InChI key
OZAIFHULBGXAKX-VAWYXSNFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Azobisisobutyronitrile (AIBN) is an azo-compound and is widely used as a free radical initiator. This compound has labile carbon-nitrogen covalent bond which undergoes homolytic scission under thermal, chemical or photochemical conditions producing free radicals. They are useful in many reactions like halogenation, polymerisation of vinyl monomers, grafting reactions, curing of rubbers and unsaturated polymers and cross-linking of polyolefins.
애플리케이션
- Used as an initiator in the synthesis of highly cross-linked Poly(divinylbenzene) (PDVB) polymers.
- Used as an initiator in the polymerization process of 2-hydroxyethyl methacrylate (HEMA).
특징 및 장점
Decomposes unimolecularly at good rates without much variation from one solvent to another.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
1.4 °F
Flash Point (°C)
-17 °C
이미 열람한 고객
Synthesis of highly cross-linked polymers in supercritical carbon dioxide by heterogeneous polymerization.
Cooper, A. I., Hems, W. P., & Holmes, A. B.
Macromolecules, 32(7), 2156-2166 (1999)
Biomaterials based on 2-hydroxyethyl methacrylate: the influence of the initiator type
Nita, L. E., Chiriac, A. P., Nistor, M. T., & Stoica, I.
Rev. Roum. Chim., 56(5), 537-543 (2011)
Selected radical azoinitiators in the synthesis of solvent-borne acrylic pressure-sensitive adhesives
Pabin-Szafko, B., Wisniewska, E., & Czech, Z.
Chemistry and Chemical Technology, 3(2), 101-106 (2009)
Initiator efficiency in radical polymerization
Walling, C.
Journal of Polymer Science, 14(74), 214-217 (1954)
Lianghui Liu et al.
Organic letters, 14(22), 5692-5695 (2012-10-31)
In the presence of a catalytic amount of radical initiator AIBN, primary amines are oxidatively coupled to imines and tertiary amines are cyanated to α-aminonitriles. These "metal-free" aerobic oxidative coupling reactions may find applications in a wide range of "green"
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.