74437
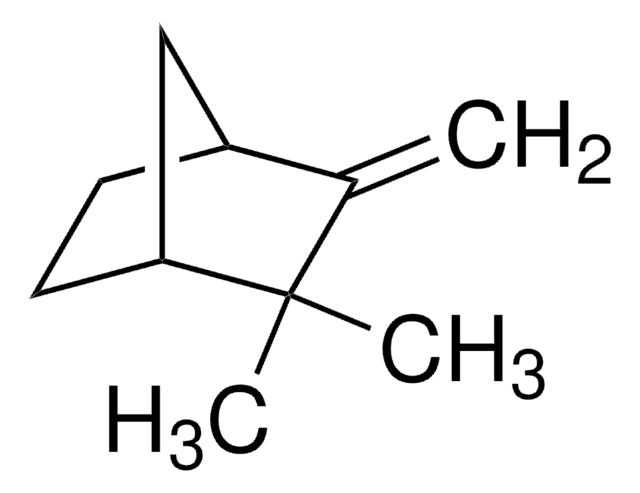
(+)-Nootkatone
≥99.0% (GC)
동의어(들):
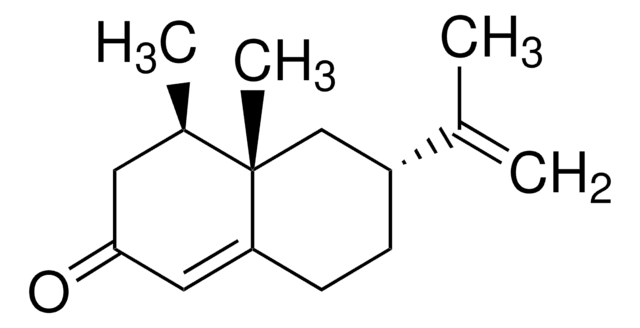
(4R,4aS,6R)-4,4a,5,6,7,8-Hexahydro-4,4a-dimethyl-6-(1-methylethenyl)-2(3H)-naphthalenone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C15H22O
CAS Number:
Molecular Weight:
218.33
Beilstein:
4676969
EC Number:
MDL number:
UNSPSC 코드:
12352205
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥99.0% (GC)
형태
crystals
광학 활성
[α]20/D +182.0±5.0°, c = 1% in ethanol
저장 조건
under inert gas (Argon)
mp
35-39 °C
작용기
ketone
저장 온도
2-8°C
SMILES string
C[C@@H]1CC(=O)C=C2CC[C@H](C[C@@]12C)C(C)=C
InChI
1S/C15H22O/c1-10(2)12-5-6-13-8-14(16)7-11(3)15(13,4)9-12/h8,11-12H,1,5-7,9H2,2-4H3/t11-,12-,15+/m1/s1
InChI key
WTOYNNBCKUYIKC-JMSVASOKSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(+)-nootkatone, a bicyclic conjugated sesquiterpene ketone with a grapefruit-like flavor, is commonly used in fragrance, food, cosmetics and pharmaceutical industry. It can be synthesized from (+)-valencene via biotransformation. (+)-nootkatone is the active ingredient responsible for the antiplatelet effect of Cyperus rotundus, a well-known oriental traditional medicine. It also shows promising efficacy against Staphylococcus aureus biofilms.
애플리케이션
(+)-Nootkatone can be used:
- In the iron(III) porphyrin catalyzed alkene aziridination reaction with organic azides under photo-irradiation condition.
- As a substrate to synthesize its corresponding triene derivative through reduction-elimination reaction using iridium catalyst.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Iridium-catalysed highly selective reduction--elimination of steroidal 4-en-3-ones to 3, 5-dienes in water
Li J, et al.
Green Chemistry, 21(8), 2088-2094 (2019)
Iron porphyrin catalysed light driven C-H bond amination and alkene aziridination with organic azides
Du Y, et al.
Chemical Science, 11(18), 4680-4686 (2020)
Katarina Cankar et al.
FEBS letters, 585(1), 178-182 (2010-12-01)
Chicory (Cichorium intybus L.), which is known to have a variety of terpene-hydroxylating activities, was screened for a P450 mono-oxygenase to convert (+)-valencene to (+)-nootkatone. A novel P450 cDNA was identified in a chicory root EST library. Co-expression of the
Robert W Behle et al.
Journal of medical entomology, 48(6), 1120-1127 (2012-01-14)
Nootkatone is a component of grapefruit oil that is toxic to the disease-vectoring tick, Ixodes scapularis Say, but unfortunately causes phytotoxicity to treated plants and has a short residual activity due to volatility. We prepared a lignin-encapsulated nootkatone formulation to
Betty C R Zhu et al.
Pest management science, 66(8), 875-878 (2010-07-06)
Research has shown that the family of grapefruit flavors called nootkatones have significant repellant and toxic effects to Formosan subterranean termites (Coptotermes formosanus Shiraki). Nineteen synthetic nootkatone derivatives, along with three commercially available nootkatone derivatives, were tested for repellent activity
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.