모든 사진(3)
About This Item
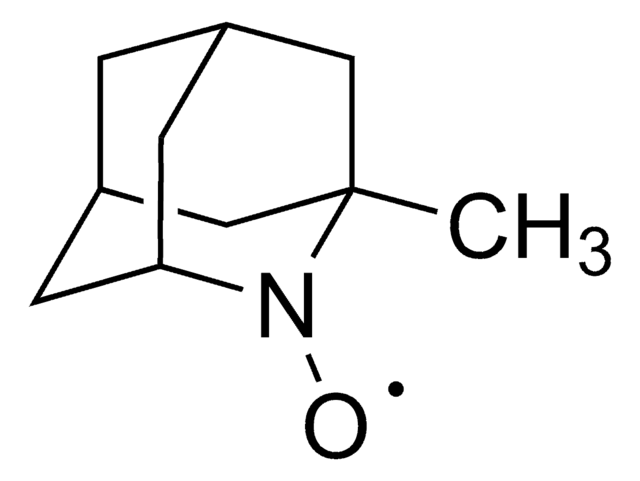
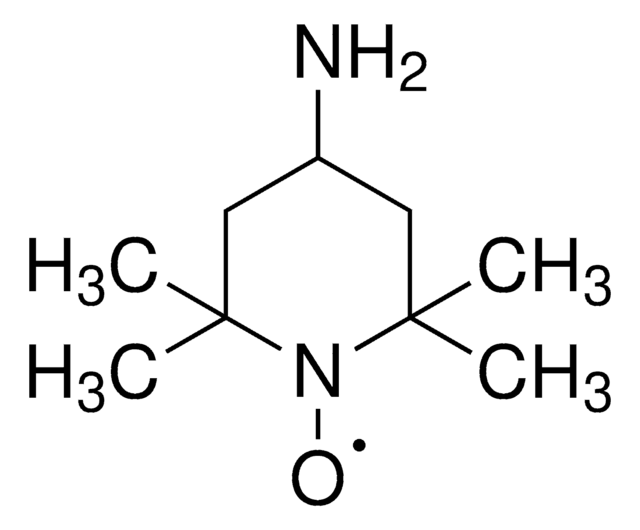
실험식(Hill 표기법):
C9H14NO
CAS Number:
Molecular Weight:
152.21
MDL number:
UNSPSC 코드:
12352000
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
90%
양식
powder
반응 적합성
reagent type: oxidant
mp
182-189 °C (D)
저장 온도
2-8°C
SMILES string
[O]N1[C@@H]2C[C@H]3C[C@@H](C2)C[C@@H]1C3
InChI
1S/C9H14NO/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-9H,1-5H2/t6-,7+,8-,9+
InChI key
BCJCJALHNXSXKE-SPJNRGJMSA-N
일반 설명
2-Azaadamantane-N-oxyl (AZADO), a stable nitroxyl radical, is widely employed as catalyst for the oxidation of alcohols.
애플리케이션
2-Azaadamantane-N-oxyl (AZADO) may be employed in the following studies:
- As catalyst for the oxidation of wood cellulose.
- As catalyst in the total synthesis of Yaku′amide A, a potential cytotoxin obtained from sponge Ceratopsion sp.
- As oxidant for the oxidation of (S)-glycidol.
이미 열람한 고객
Masatoshi Shibuya et al.
Journal of the American Chemical Society, 128(26), 8412-8413 (2006-06-29)
Development of a stable nitroxyl radical class of catalysts, 2-azaadamantane N-oxyl (AZADO) and 1-Me-AZADO, for highly efficient oxidation of alcohols is described. AZADO and 1-Me-AZADO exhibit superior catalytic proficiency to TEMPO, converting various sterically hindered alcohols to the corresponding carbonyl
Takefumi Kuranaga et al.
Journal of the American Chemical Society, 135(14), 5467-5474 (2013-03-19)
Here we report the first total synthesis and the complete stereochemical assignment of yaku'amide A. Yaku'amide A (1) was isolated from a sponge Ceratopsion sp. as an extremely potent cytotoxin. Its structure was determined except for the C4-stereochemistry in the
Ming Zhang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(10), 3937-3941 (2015-01-22)
An increased supply of scarce or inaccessible natural products is essential for the development of more sophisticated pharmaceutical agents and biological tools, and thus the development of atom-economical, step-economical and scalable processes to access these natural products is in high
Takuya Isogai et al.
Biomacromolecules, 11(6), 1593-1599 (2010-05-18)
Curdlan, amylodextrin, and regenerated cellulose fiber were subjected to electromediated oxidation with a 4-acetamido-TEMPO catalyst in a buffer at pH 6.8 without NaClO or NaClO(2). More than 90% of the C6 primary hydroxyls of Curdlan and amylodextrin were converted to
문서
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)