697400
3-Hexylthiophene-2-boronic acid pinacol ester
95%
동의어(들):
2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 3-Hexyl-2-thienylboronic acid
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C16H27BO2S
CAS Number:
Molecular Weight:
294.26
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
양식
liquid
refractive index
n20/D 1.490-1.499
density
0.983 g/mL at 25 °C
SMILES string
CCCCCCc1ccsc1B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H27BO2S/c1-6-7-8-9-10-13-11-12-20-14(13)17-18-15(2,3)16(4,5)19-17/h11-12H,6-10H2,1-5H3
InChI key
XCXAUPBHQCCWCI-UHFFFAOYSA-N
애플리케이션
Reagent used for
Reagent used in Preparation of
- Suzuki-Miyaura cross-coupling reactions
- p-type/n-type switching of ambipolar bithiazole-benzothiadiazole-based polymers in solar cells
- Hierarchical self-assembly of semiconductor functionalized peptide a-helixes and optoelectronic properties
Reagent used in Preparation of
- Photovoltaic materials, polymers, and thiophene-based compounds with photophysical, electrochemical, and fluorescent properties
- Polymer solar cells for Low band gap poly(1,4-arylene-2,5-thienylene)s with benzothiadiazole units
- Dithienothiophene-based dyes for dye-sensitized solar cells
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
>230.0 °F
Flash Point (°C)
> 110 °C
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Photophysical and Electrochemical Properties of Thiophene-Based 2-Arylpyridines
Coluccini, C.; et al.
European Journal of Organic Chemistry, 28, 5587-5598 (2011)
p/n Switching of Ambipolar Bithiazole-Benzothiadiazole-Based Polymers in Photovoltaic Cells
Balan, B.; et al.
Macromolecules, 45, 2709-2719 (2012)
Rohan J Kumar et al.
Journal of the American Chemical Society, 133(22), 8564-8573 (2011-05-17)
To determine the ability of semiconductors templated by α-helical polypeptides to form higher order structures and the charge carrier properties of the supramolecular assemblies, L-lysine was functionalized with a sexithiophene organic semiconductor unit via iterative Suzuki coupling and the click
Preparation, structure, and spectral properties of cyclophanes consisting of oligothiophene units
Tsuge, A.; et al.
Chemistry Letters (Jpn), 37, 870-871 (2008)
Photovoltaic response to structural modifications on a series of conjugated polymers based on 2-aryl-2H-benzotriazoles
Pasker, F. M.; et al.
Journal of Polymer Science Part A: Polymer Chemistry, 49, 5001-5011 (2011)
문서
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.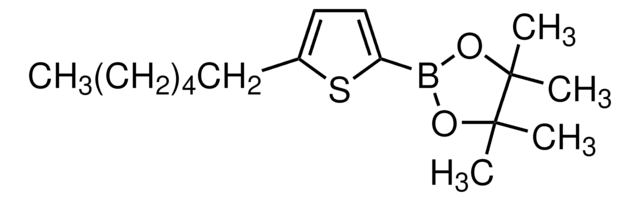

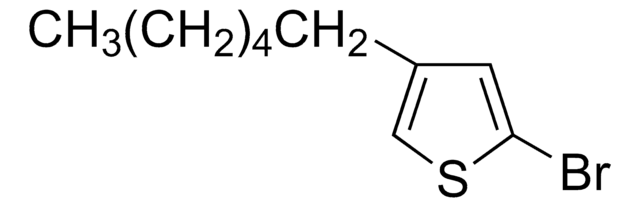
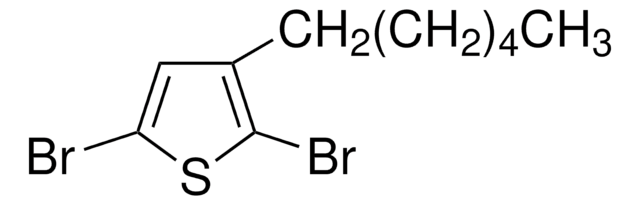

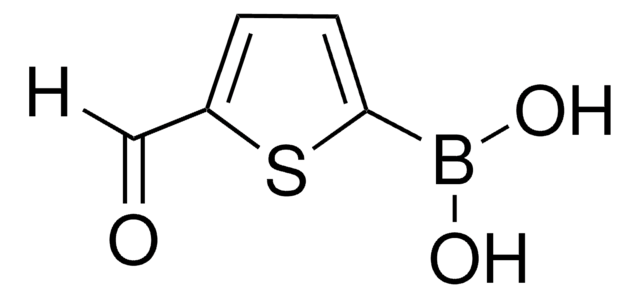
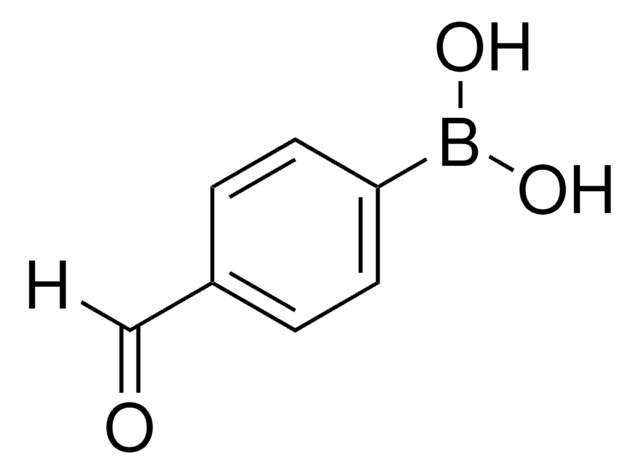

![4,7-Dibromobenzo[c]-1,2,5-thiadiazole 95%](/deepweb/assets/sigmaaldrich/product/structures/711/964/3fd3ffd1-5916-468e-a743-22f1611b5a33/640/3fd3ffd1-5916-468e-a743-22f1611b5a33.png)