추천 제품
양식
powder
Quality Level
작용기
phosphine
저장 온도
2-8°C
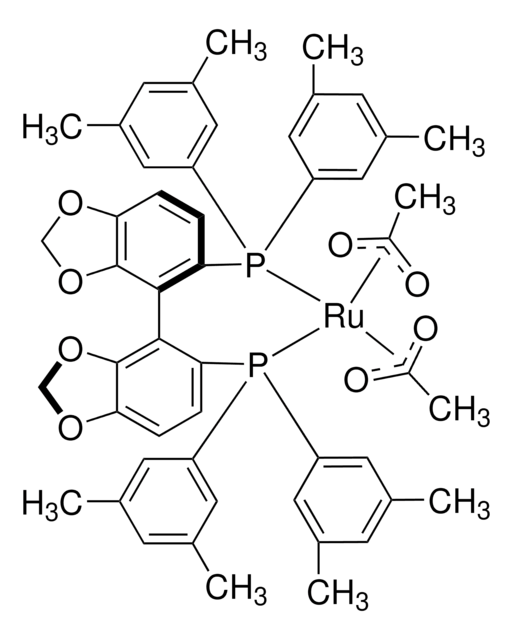
SMILES string
[Ru+2].P(c8ccccc8)(c7ccccc7)c1c(c6c(cc1)OCO6)c2c3c(ccc2P(c5ccccc5)c4ccccc4)OCO3.[O-]C(=O)C.[O-]C(=O)C
InChI
1S/C38H28O4P2.2C2H4O2.Ru/c1-5-13-27(14-6-1)43(28-15-7-2-8-16-28)33-23-21-31-37(41-25-39-31)35(33)36-34(24-22-32-38(36)42-26-40-32)44(29-17-9-3-10-18-29)30-19-11-4-12-20-30;2*1-2(3)4;/h1-24H,25-26H2;2*1H3,(H,3,4);/q;;;+2/p-2
InChI key
BHGLLIGZFQVMBJ-UHFFFAOYSA-L
애플리케이션
(S)-Ru(OAc)2(SEGPHOS®) can be used as a catalyst:
- To prepare highly chemo, enantio, and diastereoselective primary β-amino lactams by asymmetric reductive amination of racemic β-keto lactams.
- To synthesize chiral primary diarylmethylamines and sterically bulky benzylamines from diaryl and sterically hindered ketones via asymmetric reductive amination reaction.
- For the conversion of levulinic acid to optically active γ-valerolactone via asymmetric hydrogenation reaction.
법적 정보
Sold in collaboration with Takasago for research purposes only. JP Registration No. 3148136
SEGPHOS is a registered trademark of Takasago Intl. Corp.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Direct asymmetric reduction of levulinic acid to gamma-valerolactone: synthesis of a chiral platform molecule
Tukacs JM, et al.
Green Chemistry, 17(12), 5189-5195 (2015)
Dynamic Kinetic Asymmetric Reductive Amination: Synthesis of Chiral Primary ?-Amino Lactams
Lou Y, et al.
Angewandte Chemie (International ed. in English), 57(43), 14193-14197 (2018)
문서
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.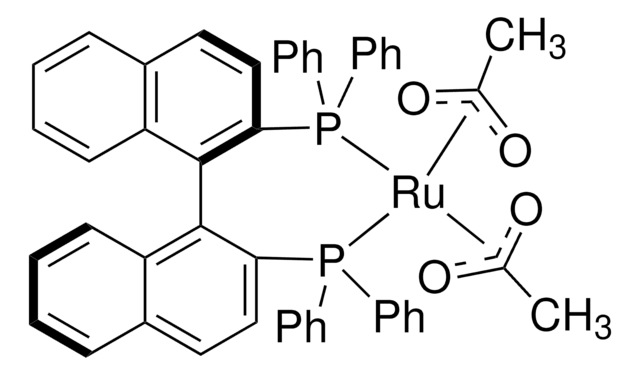
![Ru(OAc)2[(R)-dtbm-SEGPHOS®] Takasago](/deepweb/assets/sigmaaldrich/product/structures/828/527/01325f8f-b95d-42c5-8cca-81e34c63245e/640/01325f8f-b95d-42c5-8cca-81e34c63245e.png)
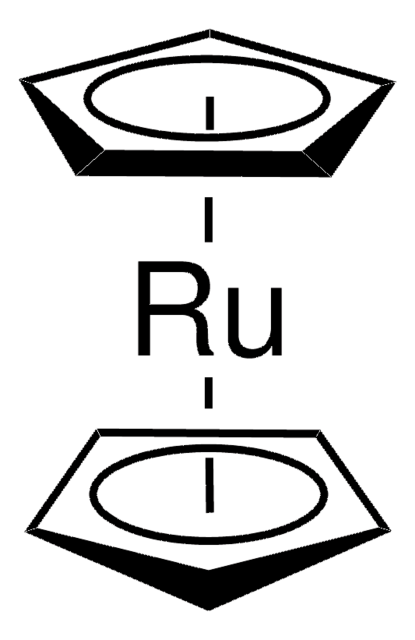
![(R)-RuCl[(p-cymene)(BINAP)]Cl](/deepweb/assets/sigmaaldrich/product/structures/244/078/7a0bdab6-11cc-4030-bbe9-4f687a6a925a/640/7a0bdab6-11cc-4030-bbe9-4f687a6a925a.png)