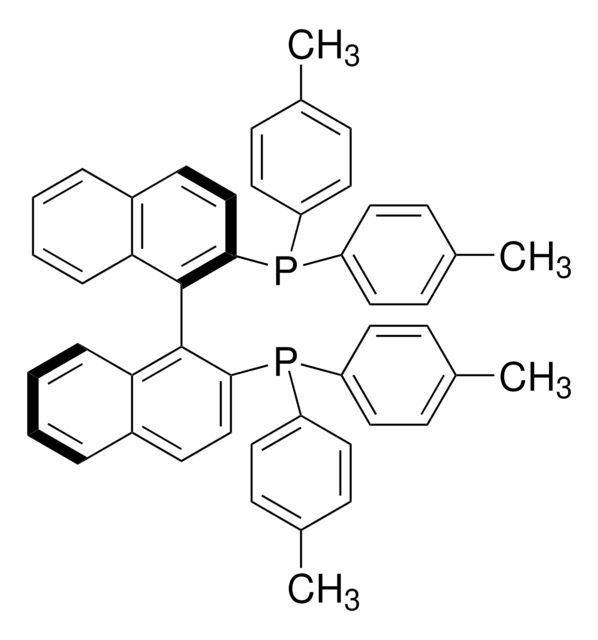
추천 제품
양식
solid
광학 활성
[α]20/D -156°, c = 0.5 in benzene
mp
250-255 °C
작용기
phosphine
SMILES string
P(c8ccc(cc8)C)(c7ccc(cc7)C)c1c(c6c(cc1)cccc6)c2c3c(ccc2P(c5ccc(cc5)C)c4ccc(cc4)C)cccc3
InChI
1S/C48H40P2/c1-33-13-23-39(24-14-33)49(40-25-15-34(2)16-26-40)45-31-21-37-9-5-7-11-43(37)47(45)48-44-12-8-6-10-38(44)22-32-46(48)50(41-27-17-35(3)18-28-41)42-29-19-36(4)20-30-42/h5-32H,1-4H3
InChI key
IOPQYDKQISFMJI-UHFFFAOYSA-N
애플리케이션
법적 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
문서
We present an article concerning BINAP/SEGPHOS® Ligands and Complexes.
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.