모든 사진(3)
About This Item
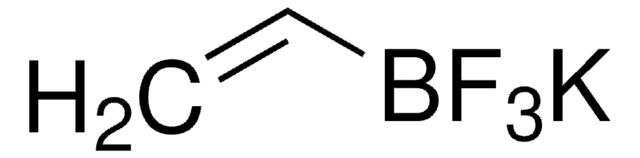
Linear Formula:
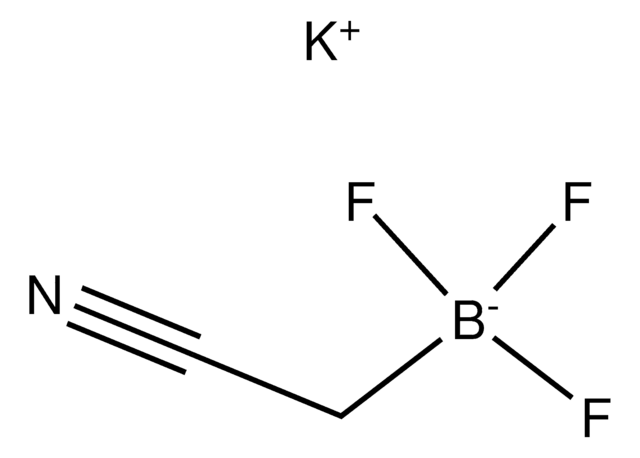
BrCH2BF3K
CAS Number:
Molecular Weight:
200.84
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
90%
양식
solid
mp
225-230 °C
작용기
bromo
SMILES string
[K+].F[B-](F)(F)CBr
InChI
1S/CH2BBrF3.K/c3-1-2(4,5)6;/h1H2;/q-1;+1
InChI key
AZDFPIRYUOCVCJ-UHFFFAOYSA-N
애플리케이션
Organotrifluoroborate involved in:
Organotrifluoroborates as versatile and stable boronic acid surrogates.
- Suzuki-Miyaura cross-coupling reactions
- Synthesis of functionalized ethyltrifluoroborates
- SN2 displacement with alkoxides
Organotrifluoroborates as versatile and stable boronic acid surrogates.
Versatile starting material for preparation of a variety of functionalized substrates for Suzuki coupling.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Gary A Molander et al.
Organic letters, 8(13), 2767-2770 (2006-06-16)
[reaction: see text] We have successfully prepared potassium azidoalkyltrifluoroborates from the corresponding halogen compounds in 94-98% yields through a nucleophilic substitution reaction with NaN(3). In the presence of various alkynes and Cu(I) as a catalyst, these azidotrifluoroborates easily formed 1,4-disubstituted
문서
These bench stable Potassium Organotrifluoroborates are useful for Suzuki-Miyaura cross-coupling reactions and have also been used for a variety of other C-C bond forming reactions. Importantly, these reagents are compatible with a wide range of functional groups and are stable to many commonly used and harsh reaction conditions.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.