674788
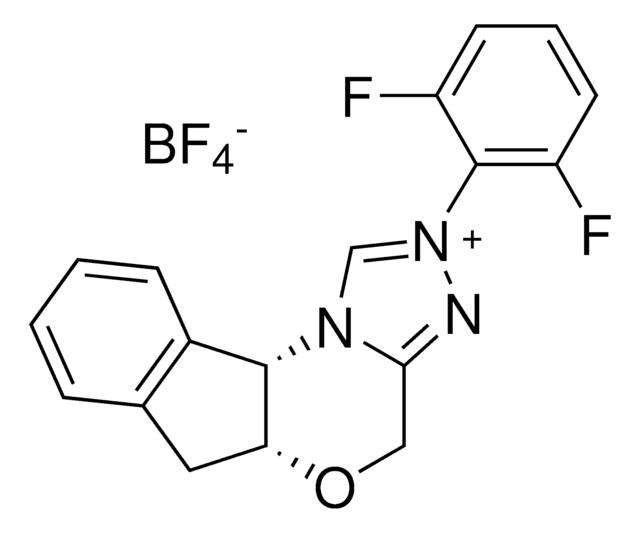
5a(R),10b(S)-5a,10b-Dihydro-2-(pentafluorophenyl)-4H,6H-indeno[2,1-b][1,2,4]triazolo[4,3-d][1,4]oxazinium tetrafluoroborate
97%
동의어(들):
2-Pentafluorophenyl-6,10b-dihydro-4H,5aH-5-oxo-3,10c-diaza-2-azoniacyclopenta[c]fluorine tetrafluoroborate
About This Item
추천 제품
Quality Level
분석
97%
형태
solid
mp
233-237 °C
작용기
ether
fluoro
저장 온도
2-8°C
SMILES string
F[B-](F)(F)F.Fc1c(F)c(F)c(c(F)c1F)-[n+]2c[n@@H]3[C@@H]4[C@@H](Cc5ccccc45)OCc3n2
InChI
1S/C18H11F5N3O.BF4/c19-12-13(20)15(22)18(16(23)14(12)21)26-7-25-11(24-26)6-27-10-5-8-3-1-2-4-9(8)17(10)25;2-1(3,4)5/h1-4,7,10,17H,5-6H2;/q+1;-1/t10-,17+;/m1./s1
InChI key
CPCMDOOTVHDRTM-CVJFODCESA-N
애플리케이션
- Benzoin reactions of aldehydes and total synthesis of a natural product named isodarparvinol B.
- Synthesis of spirocyclic oxindole-dihydropyranones by reacting α-bromo-α,β-unsaturated aldehydes with isatin derivatives.
- Synthesis of 1,4-dicarbonyl compounds through intramolecular Stetter reaction.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
문서
Rovis has demonstrated that triazolium salt in the presence of a base can act as an N-heterocyclic carbene organocatalyst in highly enantioselective intramolecular Stetter reactions.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![6,7-Dihydro-2-pentafluorophenyl-5H-pyrrolo[2,1-c]-1,2,4-triazolium tetrafluoroborate 97%](/deepweb/assets/sigmaaldrich/product/structures/338/297/58bedd1e-fa2f-4f2d-92eb-c61b56af8ded/640/58bedd1e-fa2f-4f2d-92eb-c61b56af8ded.png)
![(5aR,10bS)-5a,10b-Dihydro-2-(2,4,6-trimethylphenyl)-4H,6H-indeno[2,1-b]-1,2,4-triazolo[4,3-d]-1,4-oxazinium chloride monohydrate 93%](/deepweb/assets/sigmaaldrich/product/structures/104/483/183b49bc-426f-411b-8d11-71bbd4b81022/640/183b49bc-426f-411b-8d11-71bbd4b81022.png)

![2-Mesityl-2,5,6,7-tetrahydropyrrolo[2,1-c][1,2,4]triazol-4-ium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/267/516/0a2e9bce-0442-44c8-b912-3f3eeae583cf/640/0a2e9bce-0442-44c8-b912-3f3eeae583cf.png)
![(5aS,10bR)-5a,10b-Dihydro-2-mesityl-4H,6H-indeno[2,1-b]-1,2,4-triazolo[4,3-d]-1,4-oxazinium chloride](/deepweb/assets/sigmaaldrich/product/structures/226/284/a4e3161c-6ede-440f-8902-6af8e576a3ab/640/a4e3161c-6ede-440f-8902-6af8e576a3ab.png)
![(S)-5-Benzyl-2-mesityl-6,6-dimethyl-6,8-dihydro-5H-[1,2,4]triazolo[3,4-c][1,4]oxazin-2-ium tetrafluoroborate](/deepweb/assets/sigmaaldrich/product/structures/264/074/1f7b927e-bdc3-4352-9a96-ff81396c1618/640/1f7b927e-bdc3-4352-9a96-ff81396c1618.png)
![(5R,6S)-2-Mesityl-5,6-diphenyl-6,8-dihydro-5H-[1,2,4]triazolo[3,4-c][1,4]oxazin-2-ium tetrafluoroborate 97%](/deepweb/assets/sigmaaldrich/product/structures/219/182/9bfa803e-8970-4dd1-9cbf-9ebca2f74da2/640/9bfa803e-8970-4dd1-9cbf-9ebca2f74da2.png)