모든 사진(2)
About This Item
Linear Formula:
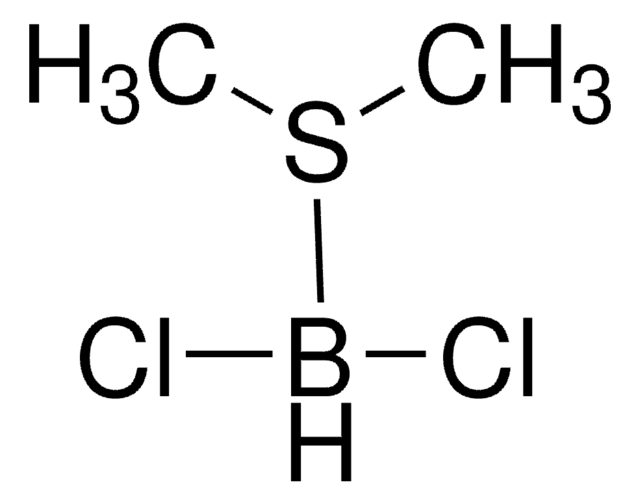
C4H8O2 · BHCl2
CAS Number:
Molecular Weight:
170.83
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
반응 적합성
reagent type: reductant
Quality Level
농도
3 M in methylene chloride
density
1.321 g/mL at 25 °C
저장 온도
2-8°C
SMILES string
Cl[BH-](Cl)[O+]1CCOCC1
InChI
1S/C4H9BCl2O2/c6-5(7)9-3-1-8-2-4-9/h5H,1-4H2
InChI key
RCKKTUSYECYDLY-UHFFFAOYSA-N
애플리케이션
Reactant for:
- Hydroboration reactions
- Preparation of alkenyl- and alkylboronic acids
Can readily substitute for common hydroborating reagents such as BH3 · THF and BMS.
법적 정보
U.S. Pat. No. 6,008,414
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
221.0 °F - closed cup
Flash Point (°C)
105 °C - closed cup
J V Kanth et al.
The Journal of organic chemistry, 66(16), 5359-5365 (2001-08-04)
Several less volatile oxygen-containing Lewis bases, such as tert-butyl methyl ether, dioxane, anisole, ethyl acetate, beta-chloroethyl ether, and monoglyme, were examined as prospective mono- and dichloroborane carriers. Dioxane, ethyl acetate, and beta-chloroethyl ether form relatively stable boron trichloride adducts, but
Kanth, J. V. B.; Brown, H. C.
Organic Letters, 1, 315-315 (1999)
Josyula, K. V. B. et al
Tetrahedron Letters, 44, 7789-7789 (2003)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.