추천 제품
제품명
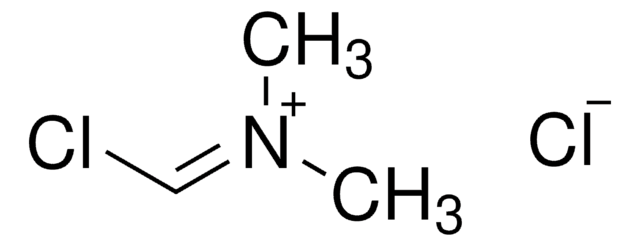
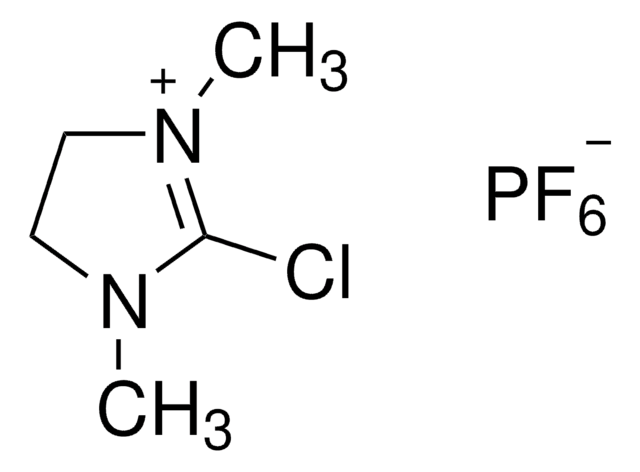
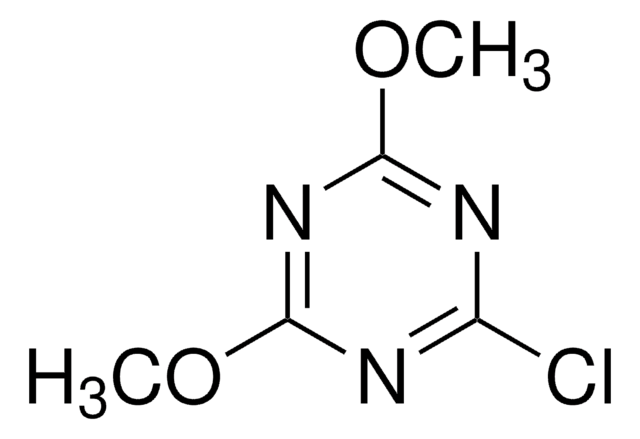
2-Chloro-1,3-dimethylimidazolinium chloride,
양식
crystalline
반응 적합성
reaction type: Coupling Reactions
mp
133-140 °C (lit.)
응용 분야
peptide synthesis
작용기
chloro
SMILES string
[Cl-].CN1CC[N+](C)=C1Cl
InChI
1S/C5H10ClN2.ClH/c1-7-3-4-8(2)5(7)6;/h3-4H2,1-2H3;1H/q+1;/p-1
InChI key
AEBBXVHGVADBHA-UHFFFAOYSA-M
애플리케이션
Activating agent in total synthesis of macroviracin A, cycloviracin B1, and cyclic silanes.
Reagent for synthesis of:
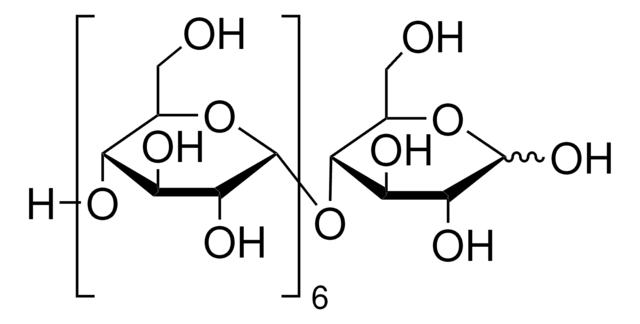
Tagged glucose as an intermediate in the synthesis of branched oligosaccharides
Fluorescent chemosensors
1,2-Diamines as inhibitors of co-activator associated arginine methyltransferase 1
Allosteric glucokinase activators
Reactant for synthesis of:
Organic azides from primary amines
Reagent for aza-Henry reactions
Tagged glucose as an intermediate in the synthesis of branched oligosaccharides
Fluorescent chemosensors
1,2-Diamines as inhibitors of co-activator associated arginine methyltransferase 1
Allosteric glucokinase activators
Reactant for synthesis of:
Organic azides from primary amines
Reagent for aza-Henry reactions
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Journal of Organometallic Chemistry, 686, 175-182 (2003)
Ken Qin et al.
Bioorganic chemistry, 94, 103391-103391 (2019-11-26)
Thermostability of monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), as a critical property of biotherapeutics, is important for their physicochemical processes, pharmacodynamics, and pharmacokinetics. Fc glycosylation of mAbs plays a crucial role in antibody functions including thermostability, however, due to
Alois Fürstner et al.
Journal of the American Chemical Society, 125(43), 13132-13142 (2003-10-23)
The first total synthesis of the antivirally active glycolipid cycloviracin B(1) (1) is described. The approach is based on a two-directional synthesis strategy which constructs the C(2)()-symmetrical macrodiolide core of the target by an efficient template-directed macrodilactonization reaction promoted by
Yanzi Gou et al.
Journal of polymer science. Part A, Polymer chemistry, 51(12), 2588-2597 (2013-06-14)
Synthetic glycopolymers are important natural oligosaccharides mimics for many biological applications. To develop glycopolymeric drugs and therapeutic agents, factors that control the receptor-ligand interaction need to be investigated. A library of well-defined glycopolymers has been prepared by the combination of
Shunya Takahashi et al.
The Journal of organic chemistry, 69(13), 4509-4515 (2004-06-19)
The C(2)-symmetric macrodiolide core 2 of an antiviral agent, macroviracin A (1), was constructed in a single step by the intermolecular macrodimerization of C(22)-hydroxy carboxylic acid 3 with 2-chloro-1,3-dimethylimidazolinium chloride and DMAP in the presence of sodium hydride (NaH). The
문서
N-Acylimidazoles were recognized in the early 1950s as reactive intermediates suitable for the acylation of amino compounds. The search for better coupling reagents than DCC led to the development of CDI (1,1’-carbonyldiimidazole) and related carbonylimidazoles.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.