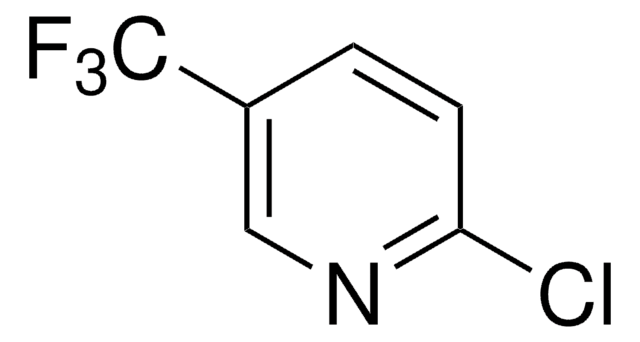
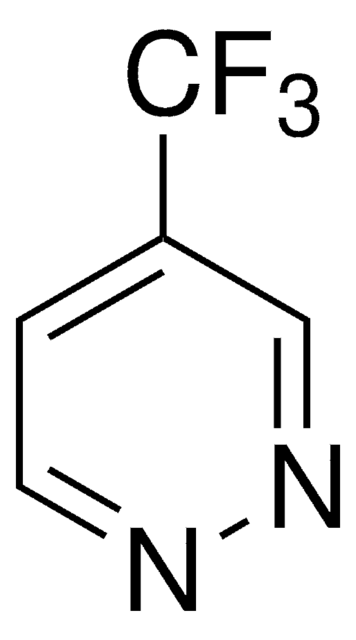
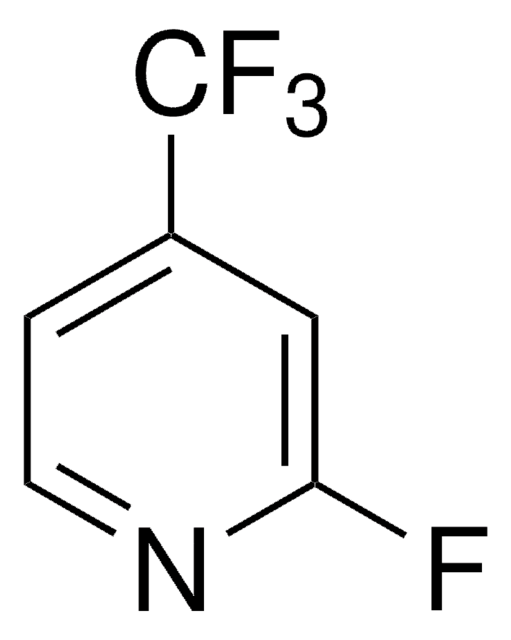
추천 제품
Quality Level
분석
97%
refractive index
n20/D 1.417 (lit.)
bp
110 °C (lit.)
density
1.27 g/mL at 25 °C (lit.)
작용기
fluoro
SMILES string
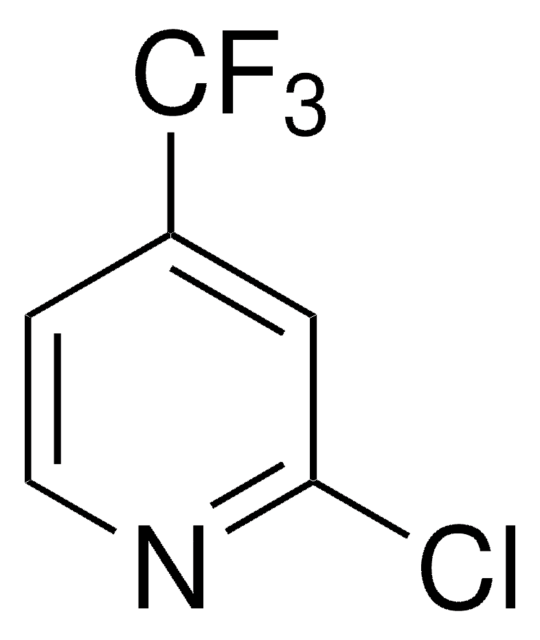
FC(F)(F)c1ccncc1
InChI
1S/C6H4F3N/c7-6(8,9)5-1-3-10-4-2-5/h1-4H
InChI key
IIYVNMXPYWIJBL-UHFFFAOYSA-N
일반 설명
4-(Trifluoromethyl)pyridine is a pyridine derivative. It can be prepared by trifluoromethylation of 4-iodobenzene.
애플리케이션
4-(Trifluoromethyl)pyridine may be used in the following:
- Preparation of (trifluoromethyl)pyridyllithiums, via metalation reaction.
- Synthesis of metal-organic frameworks (MOFs).
- Synthesis of methiodide salts.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
68.0 °F - closed cup
Flash Point (°C)
20 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Manfred Schlosser et al.
Chemical Society reviews, 36(7), 1161-1172 (2007-06-20)
Pyridines carrying heterosubstituents (such as carboxy, amido, amino, alkoxy or trifluoromethyl groups or solely individual halogen atoms) can be readily and site selectively metalated. Subsequent reaction with a suitable electrophile opens rational access to a wealth of new building blocks
Fluorinated pyridine derivatives: Part 1. The synthesis of some mono-and bis-quaternary pyridine salts of potential use in the treatment of nerve agent poisoning.
Timperley CM, et al.
Journal of Fluorine Chemistry, 126(8), 1160-1165 (2005)
Enhancement of CO2/N2 selectivity in a metal-organic framework by cavity modification.
Bae YS, et al.
Journal of Materials Chemistry, 19(15), 2131-2134 (2009)
Copper-mediated trifluoromethylation of heteroaromatic compounds by trifluoromethyl sulfonium salts.
Cheng-Pan Zhang et al.
Angewandte Chemie (International ed. in English), 50(8), 1896-1900 (2011-02-18)
Malcolm E Tessensohn et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(16), 2250-2257 (2017-06-14)
The voltammetric behavior of 2,3,5,6-tetramethyl-1,4-phenylenediamine was found to be able to differentiate the hydrogen acceptor abilities of electroinactive pyridine compounds in acetonitrile. Weak and strong hydrogen acceptors were distinguished through the onset of a third oxidation process that came about
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 522910-1G | 4061832547947 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.