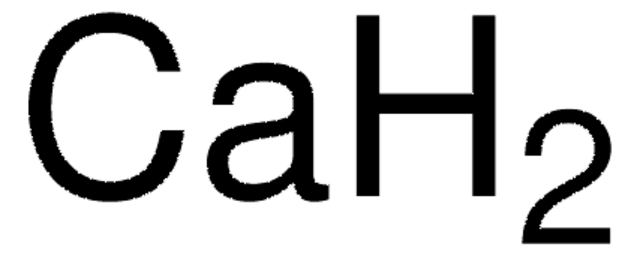추천 제품
Grade
for analytical purposes
Quality Level
분석
99.99% trace metals basis
양식
powder
반응 적합성
reagent type: reductant
환경친화적 대안 제품 특성
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
816 °C (lit.)
환경친화적 대안 카테고리
, Enabling
SMILES string
[Ca]
InChI
1S/Ca.2H
InChI key
FAQLAUHZSGTTLN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Atomic number of base material: 20
The crystal structure of calcium hydride is a slightly distorted hexagonal close packing. At elevated temperatures, calcium hydride acts as a strong reducing agent.1 Solid state reaction between hexachlorobenzene and calcium hydride has been studied in detail.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
애플리케이션
Calcium hydride (CaH2) is a metal hydride that can be used for a variety of greener applications such as:
- fabrication of hydrogen storage systems for fuel cell applications
- synthesis of porous aromatic frameworks (PAFs) for adsorption of organic pollutants
- formation of high temperature superconductors
Calcium hydride is widely used as a drying agent for organic solvents.1 It has been used to dry ε-caprolactone,benzene and toluene.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Water-react 1
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
The crystal structure of calcium hydride.
Bergsma J and Loopstra BO
Acta Crystallographica, 15(1), 92-93 (1962)
Reversible hydrogen storage in the lithium borohydride?calcium hydride coupled system
Pinkerton FE and Meyer MS
Journal of alloys and compounds, 464(1-2), L1-L4 (2008)
Synthesis of a porous aromatic framework for adsorbing organic pollutants application
Ren H, et al.
Journal of Materials Chemistry, 21(28), 10348-10353 (2011)
The mechanochemical self-propagating reaction between hexachlorobenzene and calcium hydride.
Mulas G, et al.
Journal of Solid State Chemistry, 129(2), 263-270 (1997)
Sang Cheon Lee et al.
Journal of controlled release : official journal of the Controlled Release Society, 89(3), 437-446 (2003-05-10)
Polymeric micelles based on amphiphilic block copolymers of poly(2-ethyl-2-oxazoline) (PEtOz) and poly(epsilon -caprolactone) (PCL) were prepared in an aqueous phase. The loading of paclitaxel into PEtOz-PCL micelles was confirmed by 1H-NMR spectra. Paclitaxel was efficiently loaded into PEtOz-PCL micelles using
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.