491748
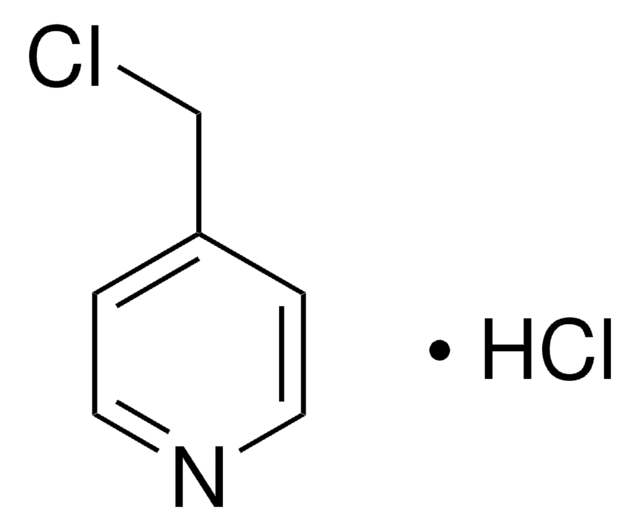
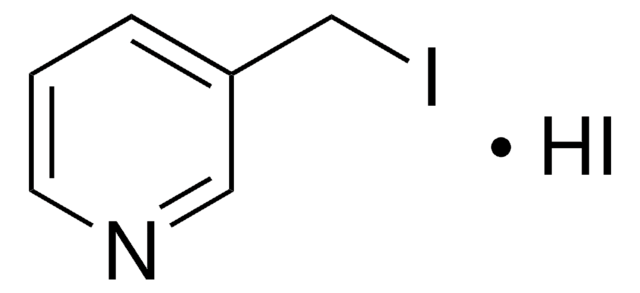
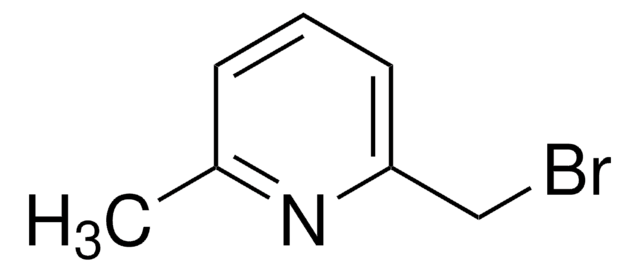
4-(Bromomethyl)pyridine hydrobromide
97%
동의어(들):
(4-Pyridyl)methyl bromide hydrobromide, 4-(Bromomethyl)pyridine monohydrobromide, 4-Picolyl bromide hydrobromide
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C6H6BrN · HBr
CAS Number:
Molecular Weight:
252.93
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
189-192 °C (lit.)
작용기
bromo
SMILES string
Br[H].BrCc1ccncc1
InChI
1S/C6H6BrN.BrH/c7-5-6-1-3-8-4-2-6;/h1-4H,5H2;1H
InChI key
VAJUUDUWDNCECT-UHFFFAOYSA-N
일반 설명
4-(Bromomethyl)pyridine hydrobromide is a substituted pyridine. It reacts with 1,2-ethanediamine and 1,3-propanediamine to form the corresponding diamines.
애플리케이션
4-(Bromomethyl)pyridine hydrobromide may be used in the preparation of:
- 3-(4-pyridylmethyl)-2′,3′-di-O-oleyl-5′-O-(4,4′-dimethoxytriphenylmethyl)uridine
- 3-(4-pyridylmethyl)-3′-O-oleyl-5′-O-(4,4-dimethoxytriphenylmethyl)-thymidine
- 1,4-bis(N-hexyl-4-pyridinium)butadiene diperchlorate
- 2-morpholin-4-yl-7-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
- 8-methyl-2-morpholin-4-yl-7-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
- 2-morpholin-4-yl-8-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Luca Simeone et al.
Molecular bioSystems, 7(11), 3075-3086 (2011-09-08)
Novel thymidine- or uridine-based nucleolipids, containing one hydrophilic oligo(ethylene glycol) chain and one or two oleic acid residues (called ToThy, HoThy and DoHu), have been synthesized with the aim to develop bio-compatible nanocarriers for drug delivery and/or produce pro-drugs. Microstructural
Photoinduced electron transfer in supramolecular complexes of a p-extended viologen with porphyrin monomer and dimer.
Fukuzumi S, et al.
Royal Society of Chemistry Advances, 2(9), 3741-3747 (2012)
Cristiane F da Costa et al.
Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 63(1), 40-42 (2008-02-12)
We report in this work the preparation and the in vitro antileishmanial activity of a series of long chains N-monoalkylated diamines and two pyridinediamine derivatives. Several compounds, tested for their in vitro antiproliferative activity against Leishmania amazonensis and Leishmania chagasi
Saleh Ihmaid et al.
European journal of medicinal chemistry, 45(11), 4934-4946 (2010-08-31)
A number of new 2-amino-[5, 6, 7 and 8]-O-substituted 1,3-benzoxazines, and 2-amino 8-methyl-7-O-substituted-1,3-benzoxazines were synthesized. Thirty one new compounds were tested for their effect on collagen induced platelet aggregation and it was found that the most active compounds were 8-methyl-2-morpholin-4-yl-7-(pyridin-3-ylmethoxy)-4H-1,3-benzoxazin-4-one
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.