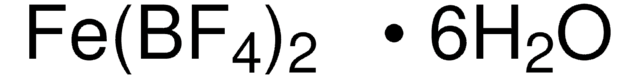482358
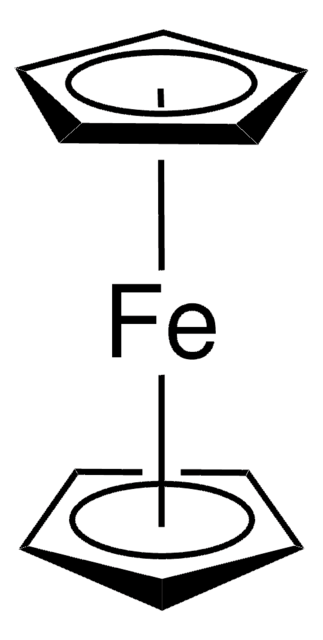
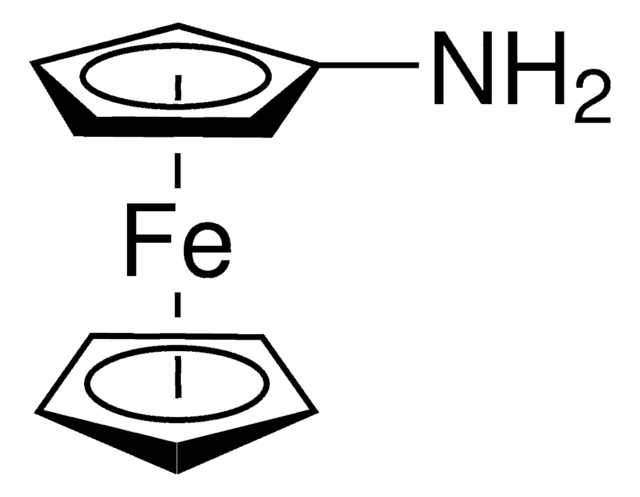
Ferrocenium tetrafluoroborate
technical grade
동의어(들):
Bis(cyclopentadienyl)iron tetrafluoroborate, Dicyclopentadienyliron fluoborate, Dicyclopentadienyliron tetrafluoroborate
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C10H10BF4Fe
CAS Number:
Molecular Weight:
272.84
MDL number:
UNSPSC 코드:
12161600
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
technical grade
반응 적합성
core: iron
reagent type: catalyst
mp
178 °C (dec.) (lit.)
SMILES string
[Fe+].F[B-](F)(F)F.[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2
InChI
1S/2C5H5.BF4.Fe/c2*1-2-4-5-3-1;2-1(3,4)5;/h2*1-5H;;/q;;-1;+1
InChI key
ZSPXIHLQPWVOQR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Ferrocenium tetrafluoroborate [FeCp2][BF4] is one of the commonly employed ferrocenium salts within chemical synthesis. Ferrocenium salts are commonly used as Lewis acid catalysts, one-electron oxidants, and electron donors.
애플리케이션
Ferrocenium tetrafluoroborate serves as:
- an oxidizing agent in the synthesis of the monocationic Co(II)complex [CpCo(azpy)]+
- a Lewis acid catalyst in epoxide ring opening and to activatethe carbonyl group for addition or cycloadditions reactions
- an oxidizing agent when used in conjuntion with a Cl-source
- a reversible redox reagent between stannole dianion and bistannole-1,2-dianion
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
R F Anderson et al.
The Journal of biological chemistry, 275(40), 30781-30786 (2000-06-22)
Trimethylamine dehydrogenase from the pseudomonad Methylophilus methylotrophus has been examined using the technique of pulse radiolysis to rapidly introduce a single reducing equivalent into the enzyme. Using enzyme that has had its iron-sulfur center rendered redox-inert by prior reaction with
Ralf Warratz et al.
Inorganic chemistry, 48(8), 3591-3607 (2009-03-27)
Ferrocene-ferrocenium dimers exhibit a double-peak intervalence charge-transfer (IVCT) band in the NIR/MIR region, which is analyzed in terms of a four-level, two-mode vibronic coupling configuration interaction (VCCI) scheme. Besides providing satisfactory fits of the measured spectra, the model also gives
T David Harris et al.
Chemical communications (Cambridge, England), 47(22), 6344-6346 (2011-05-07)
Oxidation of the nominally all-ferrous hexanuclear cluster ((H)L)(2)Fe(6) with six equivalents of ferrocenium in the presence of bromide ions results in a six-electron oxidation of the Fe(6) core to afford the nominally all-ferric cluster ((H)L)(2)Fe(6)Br(6). The hexabromide cluster is also
Songbo Xu et al.
Organic & biomolecular chemistry, 5(3), 558-568 (2007-01-26)
Various calix[4]arene derivatives, fixed in the cone conformation by decylether groups and functionalized at their wide rim by urea residues, were synthesized. In two compounds (,) sulfur functions were attached to the urea groups via different spacers in order to
Mutlu Sönmez Celebi et al.
Talanta, 78(2), 405-409 (2009-02-11)
A new surface based on poly(vinylferrocenium) (PVF(+))-modified platinum electrode was developed for determination of Hg(2+) ions in aqueous solutions. The polymer was electrodeposited on platinum electrode by constant potential electrolysis as PVF(+)ClO(4)(-). Cl(-) ions were then attached to the polymer
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.