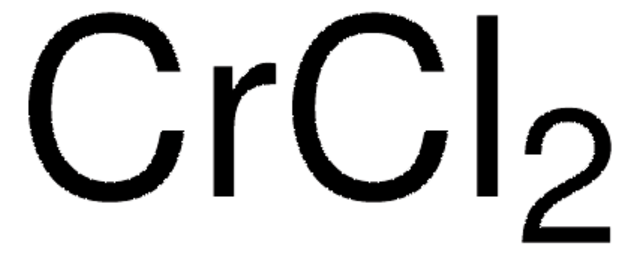451193
Nickel(II) chloride
anhydrous, powder, 99.99% trace metals basis
동의어(들):
Nickel chloride, Nickel dichloride, Nickel dichloride (NiCl2 ), Nickelous chloride
About This Item
추천 제품
Grade
anhydrous
Quality Level
분석
99.99% trace metals basis
양식
powder
불순물
≤150.0 ppm Trace Metal Analysis
density
3.55 g/mL at 25 °C (lit.)
응용 분야
battery manufacturing
SMILES string
Cl[Ni]Cl
InChI
1S/2ClH.Ni/h2*1H;/q;;+2/p-2
InChI key
QMMRZOWCJAIUJA-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
특징 및 장점
신호어
Danger
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A Inhalation - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
표적 기관
Lungs
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
문서
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Tools for Performing ATRP
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
프로토콜
gc-analysis-of-a-37-component-fame-mix-g004278
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 451193-25G | 4061838140173 |
| 451193-5G | 4061832324111 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.