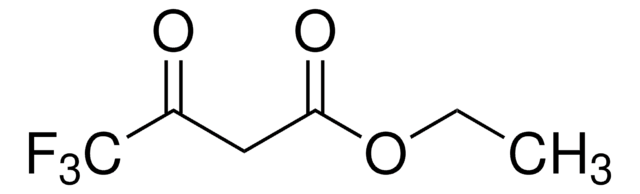
추천 제품
Quality Level
분석
96%
형태
liquid
refractive index
n20/D 1.429 (lit.)
bp
80-82 °C/1 mmHg (lit.)
density
1.235 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
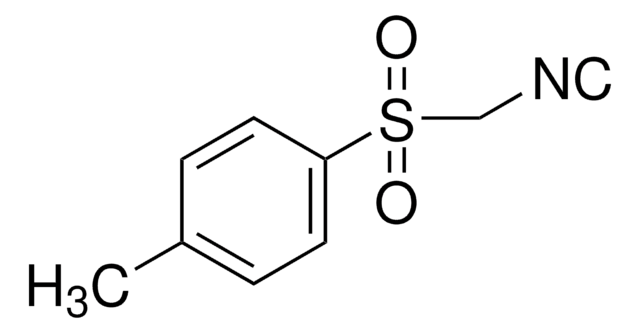
CCO\C=C(\C(=O)OCC)C(=O)C(F)(F)F
InChI
1S/C9H11F3O4/c1-3-15-5-6(8(14)16-4-2)7(13)9(10,11)12/h5H,3-4H2,1-2H3/b6-5+
InChI key
XNGGOXOLHQANRB-AATRIKPKSA-N
일반 설명
Ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate has been reported to participate in the microwave-assisted synthesis of ethyl 1-[4-(2,3,3-trichloroacrylamido)phenyl]-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate.
애플리케이션
Ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate may be employed as a starting reagent for the synthesis of 1-methyl-3-trifluoromethyl-1H-pyrazole-4- carboxylic acid.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
219.2 °F - closed cup
Flash Point (°C)
104.00 °C - closed cup
개인 보호 장비
Eyeshields, Gloves
L Sansebastiano et al.
Farmaco (Societa chimica italiana : 1989), 48(3), 335-355 (1993-03-01)
The synthesis of ethyl or methyl 4-substituted or unsubstituted 2-methylthio-5-pyrimidinecarboxylates 3 a-i and 8 o mainly by reaction of ethyl or methyl 2-dimethylaminomethylene-3-oxoalkanoates with 2-methylisothiourea is described. Also some ethyl 2-substituted (NH2, CH3, C6H5) 4-trifluoromethyl-5-pyrimidinecarboxylates were prepared. Some of the
P J Sanfilippo et al.
Journal of medicinal chemistry, 38(1), 34-41 (1995-01-06)
The synthesis and biological activity of novel thiazole-based heterocycles as inhibitors of thrombin-induced human platelet aggregation are described. Further evaluation of selected compounds show they inhibit platelet aggregation as stimulated by a variety of agonists. The more active compounds also
R D Franz
AAPS pharmSci, 3(2), E10-E10 (2001-12-14)
The changes in the physiochemical properties accompanying the substitution of a phosphonic acid group for a carboxylic acid group on various heterocyclic platforms was determined. A series of low molecular weight heterocyclic carboxylic and phosphonic acids was prepared, and the
L Mosti et al.
Farmaco (Societa chimica italiana : 1989), 47(4), 427-437 (1992-04-01)
The synthesis of ethyl or methyl esters of 5-cyano-1,6-dihydro-6-oxo-3- pyridinecarboxylic acids carrying as 2-substituent a polar group such as CO2C2H5, (CH2)2CO2CH3, (CH2)3CO2C2H5, CH2OCH3, or CF3 group is described. Also 2-[5-cyano-1,6-dihydro-2-(1,1-dimethylethyl)-6-oxo-3-pyridyl]-2- oxoacetic acid and 2,5,6,8-tetrahydro-2,5-dioxo-1H-thiopyrano[3,4-b]pyridine-3-carbon itrile were prepared. Nearly all the
European Journal of Medicinal Chemistry, 28, 853-853 (1993)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.