432725
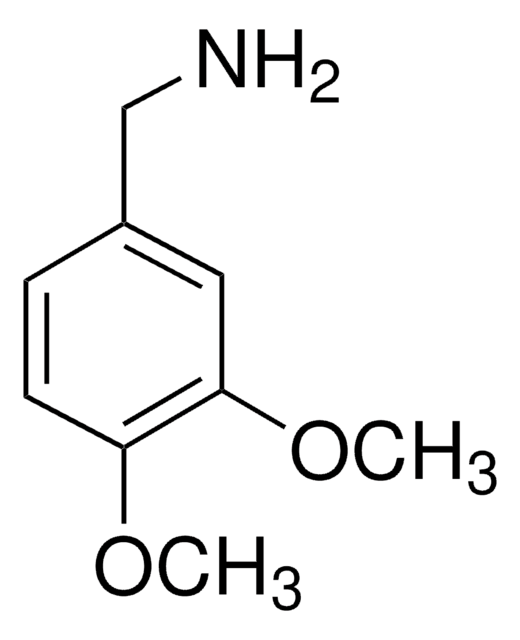
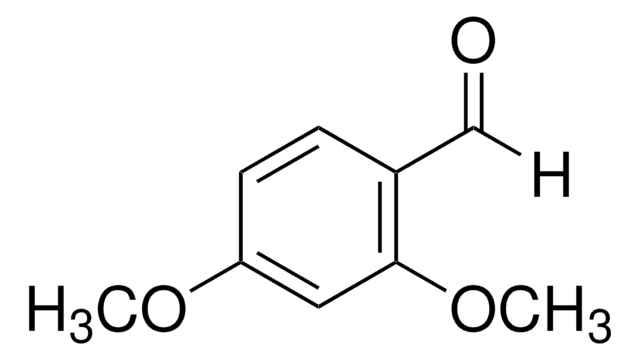
2,4-Dimethoxybenzylamine
98%
동의어(들):
(2,4-Dimethoxyphenyl)methanamine, 1-(2,4-Dimethoxyphenyl)methanamine, 2,4-Dimethoxybenzenemethanamine, 2,4-Dimethyloxybenzylamine, [(2,4-Dimethoxyphenyl)methyl]amine
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
(CH3O)2C6H3CH2NH2
CAS Number:
Molecular Weight:
167.21
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
refractive index
n20/D 1.549 (lit.)
bp
140 °C/1 mmHg (lit.)
density
1.113 g/mL at 25 °C (lit.)
작용기
amine
SMILES string
COc1ccc(CN)c(OC)c1
InChI
1S/C9H13NO2/c1-11-8-4-3-7(6-10)9(5-8)12-2/h3-5H,6,10H2,1-2H3
InChI key
QOWBXWFYRXSBAS-UHFFFAOYSA-N
일반 설명
2,4-Dimethoxybenzylamine can be preprepared by reduction (NaBH4 , BF3.OEt2, THF) of 2,4-dimethoxybenzonitrile.
애플리케이션
2,4-Dimethoxybenzylamine is an amine nucleophile used to investigate the 1,4- reactivity of 5-bromo-2-indene-1-one. It may be used in the following studies:
- As an ammonia equivalent in the concise synthesis of a series of 2,4,5-trisubstituted oxazoles, via a tandem Ugi/Robinson-Gabriel reaction sequence.
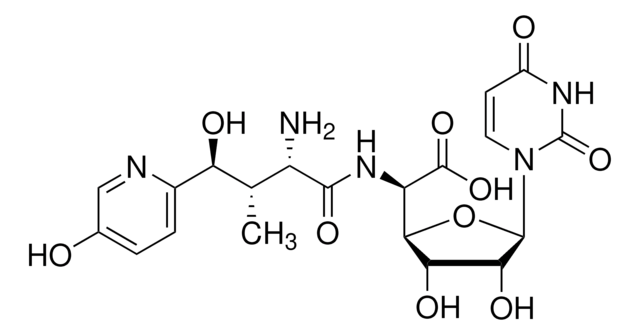
- Total synthesis of (-)-muraymycin (MRY) D2 and its epimer, the antibacterial nucleoside natural product.
- Two-step synthesis of amide derivatives of uracil polyoxin C (UPOC) methyl ester using the Ugi reaction.
- Synthesis of N-hydroxythiourea.
- Synthesis of anti-HIV-1 agents.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
M Sato et al.
Journal of medicinal chemistry, 19(2), 336-337 (1976-02-01)
The synthesis of the title compound (1) was accomplished by the conversion of 2,4-dimethoxybenzylamine (2) into an isothiocyanate (3) using thiocarbonyl diimidazole. Treatment of 3 with hydroxylamine and removal of the DMB group with trifluoroacetic acid gave 1. N-Hydroxythiourea (1)
Arthur Y Shaw et al.
Tetrahedron letters, 53(15), 1998-2000 (2013-04-06)
This Letter discloses a novel concise synthesis of a series of 2,4,5-trisubstituted oxazoles via a tandem Ugi/Robinson-Gabriel sequence. Herein, 2,4-dimethoxybenzylamine
Tetsuya Tanino et al.
The Journal of organic chemistry, 75(5), 1366-1377 (2010-02-11)
Full details of the first total synthesis of (-)-muraymycin (MRY) D2 and its epimer, the antibacterial nucleoside natural product, are described. Key strategic elements of the approach include the preparation of the urea dipeptide moiety found in the muraymycins containing
Tetrahedron Letters, 47, 8459-8459 (2006)
Keith J Stanger et al.
Journal of combinatorial chemistry, 8(3), 435-439 (2006-05-09)
We describe parallel/combinatorial, solid-phase, supported synthesis of diverse hydroxamates using a common intermediate, an N-derivatized, O-linked hydroxylamine. The method allows the concurrent synthesis of both N-alkyl and N-H hydroxamates and is compatible with a wide range of chemical transformations. The
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.