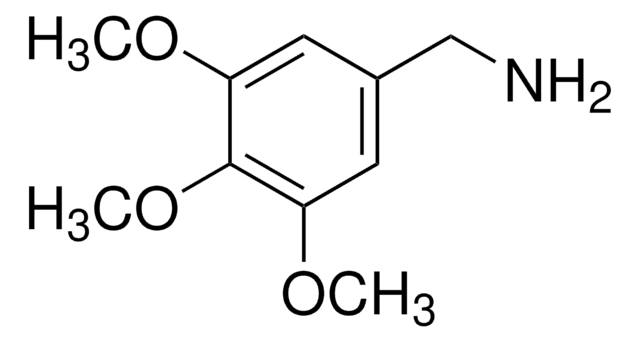
추천 제품
분석
97%
refractive index
n20/D 1.556 (lit.)
bp
281-284 °C (lit.)
density
1.109 g/mL at 25 °C (lit.)
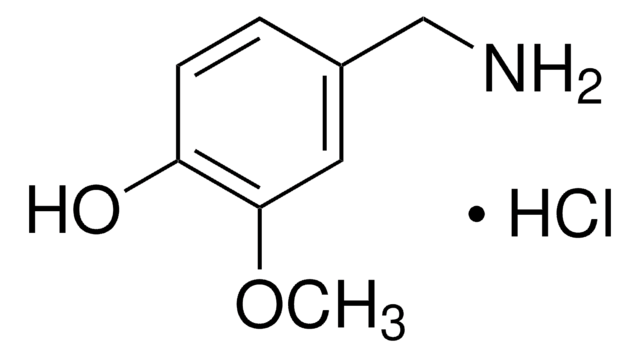
SMILES string
COc1ccc(CN)cc1OC
InChI
1S/C9H13NO2/c1-11-8-4-3-7(6-10)5-9(8)12-2/h3-5H,6,10H2,1-2H3
InChI key
DIVNUTGTTIRPQA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
<ul>
<li><strong>Fluorescence Derivatization for Serotonin Determination:</strong> 3,4-Dimethoxybenzylamine is employed as a sensitive pre-column fluorescence derivatization reagent for measuring serotonin levels in human platelet-poor plasma, enhancing detection sensitivity and specificity (Ishida et al., 1997).</li>
<li><strong>Spectrofluorimetric Analysis of 5-Hydroxyindoles:</strong> This compound serves as a selective fluorogenic reagent for the spectrofluorimetric determination of 5-hydroxyindoles, contributing to accurate and selective analysis techniques in clinical and biochemical studies (Ishida et al., 1991).</li>
</ul>
<li><strong>Fluorescence Derivatization for Serotonin Determination:</strong> 3,4-Dimethoxybenzylamine is employed as a sensitive pre-column fluorescence derivatization reagent for measuring serotonin levels in human platelet-poor plasma, enhancing detection sensitivity and specificity (Ishida et al., 1997).</li>
<li><strong>Spectrofluorimetric Analysis of 5-Hydroxyindoles:</strong> This compound serves as a selective fluorogenic reagent for the spectrofluorimetric determination of 5-hydroxyindoles, contributing to accurate and selective analysis techniques in clinical and biochemical studies (Ishida et al., 1991).</li>
</ul>
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
A M Feigin et al.
Neuroreport, 6(16), 2134-2136 (1995-11-13)
The irritating, pungent compound, capsaicin (10-20 microM), induces the formation of non-selective ion channels with a wide variety of conductances in protein-free lipid bilayers form from a mixture of zwitterionic phospholipids. The channel-forming activity of capsaicin and four of its
J Ishida et al.
Journal of chromatography. B, Biomedical sciences and applications, 692(1), 31-36 (1997-04-25)
3,4-Dimethoxybenzylamine is shown to be a highly sensitive pre-column fluorescence derivatization reagent for the determination of serotonin in plasma by high-performance liquid chromatography. The reagent reacts selectively with 5-hydroxyindoles including serotonin in slightly alkaline media in the presence of potassium
J Ishida et al.
The Analyst, 116(3), 301-304 (1991-03-01)
A spectrofluorimetric method has been developed for the sensitive and selective determination of 5-hydroxyindoles; the method is based on the reaction of 5-hydroxyindoles in a weakly alkaline solution (pH 9.0) with aromatic methylamines in the presence of potassium hexacyanoferrate(III) and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.