432032
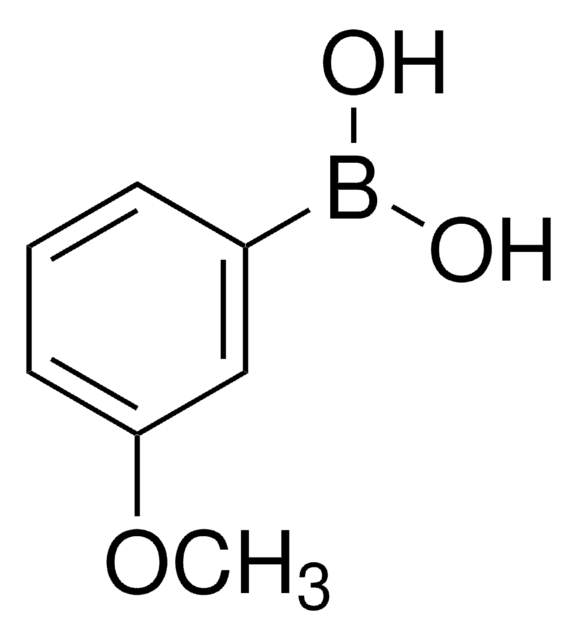
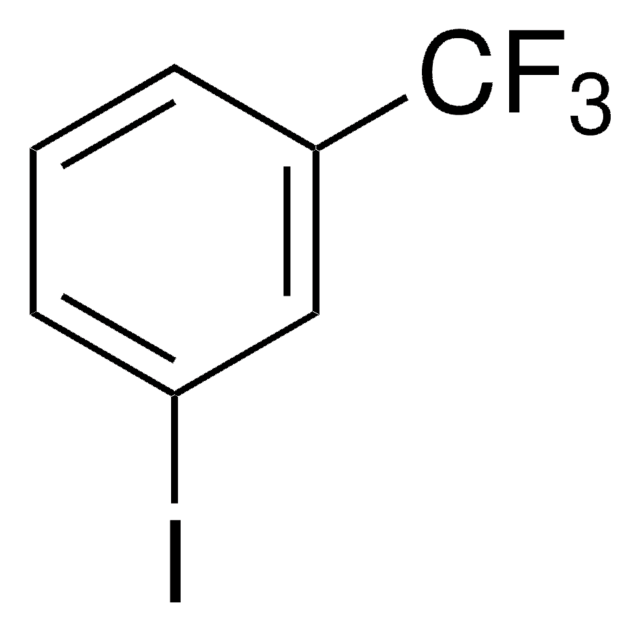
3-(Trifluoromethyl)phenylboronic acid
≥95%
동의어(들):
3-(Trifluoromethyl)benzeneboronic acid, a,a,a-Trifluoro-m-tolueneboronic acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
CF3C6H4B(OH)2
CAS Number:
Molecular Weight:
189.93
Beilstein:
6084746
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.06
추천 제품
분석
≥95%
mp
163-166 °C (lit.)
작용기
fluoro
SMILES string
OB(O)c1cccc(c1)C(F)(F)F
InChI
1S/C7H6BF3O2/c9-7(10,11)5-2-1-3-6(4-5)8(12)13/h1-4,12-13H
InChI key
WOAORAPRPVIATR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Reactant involved in:
- Suzuki-Miyaura cross-coupling reactions
- Aerobic oxidative cross-coupling
- Microwave-assisted Petasis reactions
- Rhodium-catalyzed addition reactions
- Syntehsis of biologically active molecules
기타 정보
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Anna Minkkilä et al.
Journal of medicinal chemistry, 51(22), 7057-7060 (2008-11-06)
A series of commercial phenyl-, heteroaryl-, alkyl-, and alkenylboronic acids were evaluated for their FAAH and MGL inhibitory activities. The compounds were generally selective for FAAH, with IC50 in the nanomolar or low-micromolar range. Eight of these compounds inhibited MGL
Gözde Murat Saltan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 188, 372-381 (2017-08-02)
In an approach to develop efficient organic optoelectronic devices to be used in light-driven systems, a series of three thiophene linked benzimidazole conjugates were synthesized and characterized. The combination of two thiophene rings to a benzimidazole core decorated with different
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.