426369
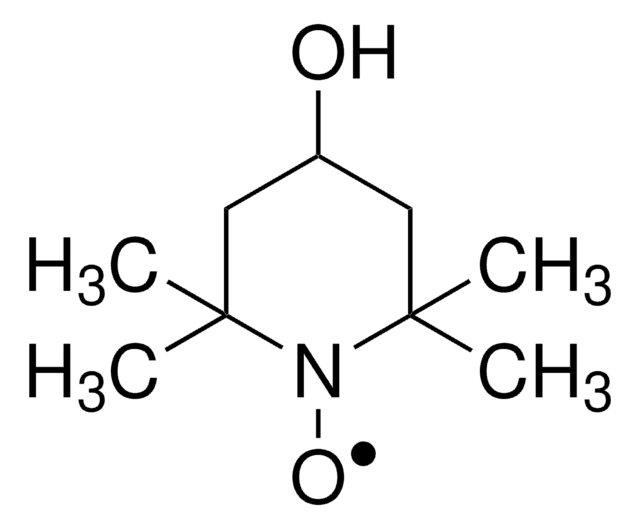
TEMPO
purified by sublimation, 99%
동의어(들):
2,2,6,6-Tetramethylpiperidine 1-oxyl, 2,2,6,6-Tetramethyl-1-piperidinyloxy, free radical, TEMPO
About This Item
추천 제품
분석
99%
양식
solid
정제법
sublimation
반응 적합성
reagent type: oxidant
mp
36-38 °C (lit.)
저장 온도
2-8°C
SMILES string
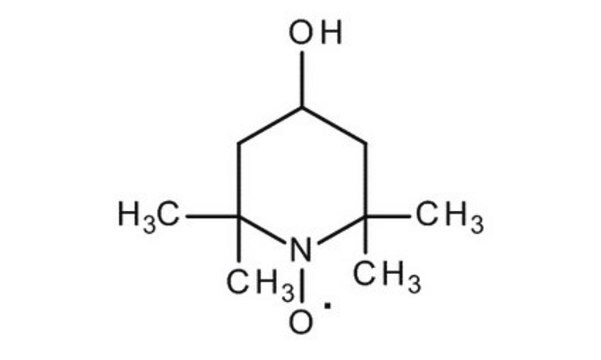
CC1(C)CCCC(C)(C)N1[O]
InChI
1S/C9H18NO/c1-8(2)6-5-7-9(3,4)10(8)11/h5-7H2,1-4H3
InChI key
QYTDEUPAUMOIOP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1C
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point (°F)
152.6 °F - closed cup
Flash Point (°C)
67 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
문서
Block Copolymer Synthesis Using a Nitroxide-mediated Radical Polymerization (NMP) Approach
Block copolymer synthesis using a commercially available nitroxide-mediated radical polymerization (NMP) initiator
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Tools for Performing ATRP
프로토콜
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Sigma-Aldrich presents an article about the typical procedures for polymerizing via ATRP, which demonstrates that in the following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.