407240
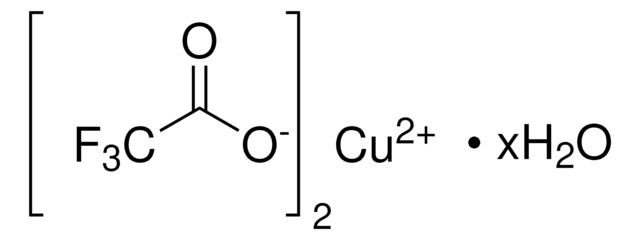
Copper(I) trifluoromethanesulfonate benzene complex
technical grade, 90%
동의어(들):
Copper(I) triflate benzene complex, Cuprous trifluoromethanesulfonate benzene complex, Trifluoromethanesulfonic acid copper(I) salt benzene complex
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
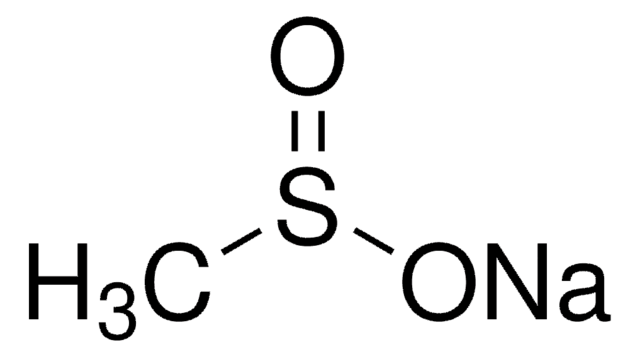
Linear Formula:
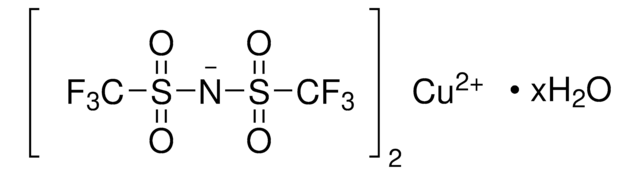
(CF3SO3Cu)2 · C6H6
CAS Number:
Molecular Weight:
503.34
EC Number:
MDL number:
UNSPSC 코드:
12161600
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
technical grade
분석
90%
양식
powder
반응 적합성
core: copper
reagent type: catalyst
mp
160 °C (dec.) (lit.)
SMILES string
[Cu+].[Cu+].c1ccccc1.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/C6H6.2CHF3O3S.2Cu/c1-2-4-6-5-3-1;2*2-1(3,4)8(5,6)7;;/h1-6H;2*(H,5,6,7);;/q;;;2*+1/p-2
InChI key
GNXZWVVAAMVOJY-UHFFFAOYSA-L
애플리케이션
Copper(I) trifluoromethanesulfonate benzene complex can be used as a catalyst:
It can also be used in combination with amino acid-based chiral phosphine ligands to catalyze asymmetric conjugate additions of alkylzincs to acyclic α,β-unsaturated ketones, affording β-alkylcarbonyls in high yield and with excellent enantioselectivity.
- To synthesize enol-esters via copper(I) carboxylate intermediate formation.
- In the enantioselective allylic oxidation of cyclic alkenes.
- To prepare 2,5-disubstituted pyrrolidine derivatives from N-alkenyl, alkynyl and alkyl N-benzoyloxysulfonamides via the sulfonamidyl radical formation.
It can also be used in combination with amino acid-based chiral phosphine ligands to catalyze asymmetric conjugate additions of alkylzincs to acyclic α,β-unsaturated ketones, affording β-alkylcarbonyls in high yield and with excellent enantioselectivity.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Flam. Sol. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Jerome Bayardon et al.
The Journal of organic chemistry, 69(9), 3121-3128 (2004-04-24)
Various enantiopure fluorous bis(oxazolines) with fluorine content between 52.7 and 58.7% have been synthesized by a simple reaction sequence that involved the introduction of two fluorinated ponytails by alkylation of the corresponding nonfluorous bis(oxazolines). These new ligands have been used
Enantioselective total synthesis of erogorgiaene: applications of asymmetric Cu-catalyzed conjugate additions of alkylzincs to acyclic enones
Cesati RR, et al.
Journal of the American Chemical Society, 126(1), 96-101 (2004)
Synthesis of Enol Esters from Copper (I) Carboxylates Generated from Copper (I) Trifluoromethanesulfonate Benzene Complex
Lefler SR and Rose SD
Synthetic Communications, 29(21), 3805-3810 (1999)
Richard R Cesati et al.
Journal of the American Chemical Society, 126(1), 96-101 (2004-01-08)
The first enantioselective synthesis of erogorgiaene (1), an inhibitor of mycobacterium tuberculosis, is disclosed. The total synthesis highlights the utility of asymmetric conjugate additions (ACA) of alkylzincs to acyclic alpha,beta-unsaturated ketones catalyzed by peptidic phosphine ligands and (CuOTf)(2).C(6)H(6). Moreover, several
Enantiopure fluorous bis (oxazolines): Synthesis and applications in catalytic asymmetric reactions
Bayardon J and Sinou D
The Journal of Organic Chemistry, 69(9), 3121-3128 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.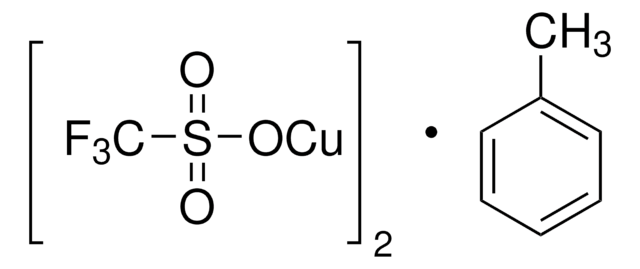
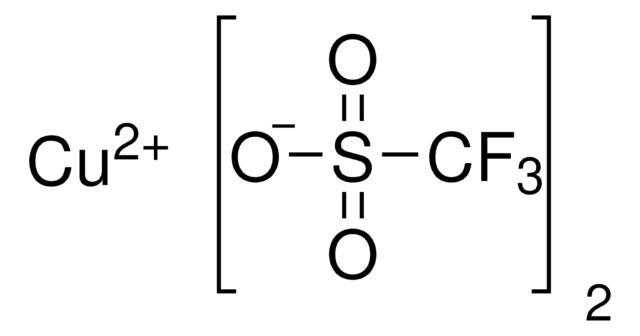

![Chloro[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I)](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)