추천 제품
분석
99%
양식
solid
광학 활성
[α]20/D +280°, c = 1 in ethyl acetate
mp
200 °C (dec.) (lit.)
작용기
ether
hydroxyl
ketal
SMILES string
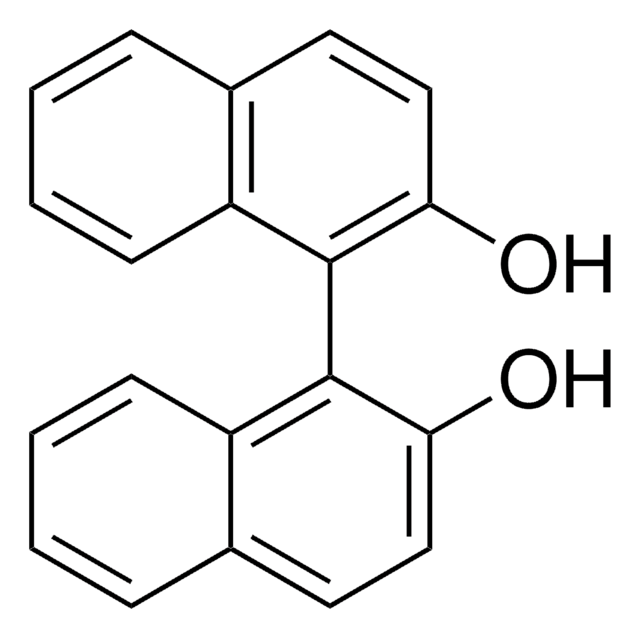
CC1(C)O[C@@H]([C@H](O1)C(O)(c2cccc3ccccc23)c4cccc5ccccc45)C(O)(c6cccc7ccccc67)c8cccc9ccccc89
InChI
1S/C47H38O4/c1-45(2)50-43(46(48,39-27-11-19-31-15-3-7-23-35(31)39)40-28-12-20-32-16-4-8-24-36(32)40)44(51-45)47(49,41-29-13-21-33-17-5-9-25-37(33)41)42-30-14-22-34-18-6-10-26-38(34)42/h3-30,43-44,48-49H,1-2H3/t43-,44-/m0/s1
InChI key
WTZVNZRNIOJACO-CXNSMIOJSA-N
애플리케이션
- For the highly enantioselective addition of primary alkyl Grignards to ketones.
- As an organocatalyst for the activation of carbonyl functionality in vinylogous addition reaction of an aldehyde.
- As a chiral dopant in the preparation of cholesteric liquid crystal (CLC) having an aggregation-induced-emission dye.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
문서
The chiral auxiliaries TADDOLs (α,α,α,α-tetraaryl-1,3-dioxolane-4,5- dimethanols) developed by Seebach's group have found numerous applications in asymmetric synthesis ranging from utilization as stoichiometric chiral reagents or in Lewis acid mediated reactions, to roles in catalytic hydrogenation and stereoregular metathesis polymerization.
Apart from numerous examples using TADDOLs in metal-catalyzed asymmetric reactions, Rawal recently reported that TADDOLs could be used as Brønsted acid organocatalysts in highly stereoselective hetero-Diels–Alder reactions.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.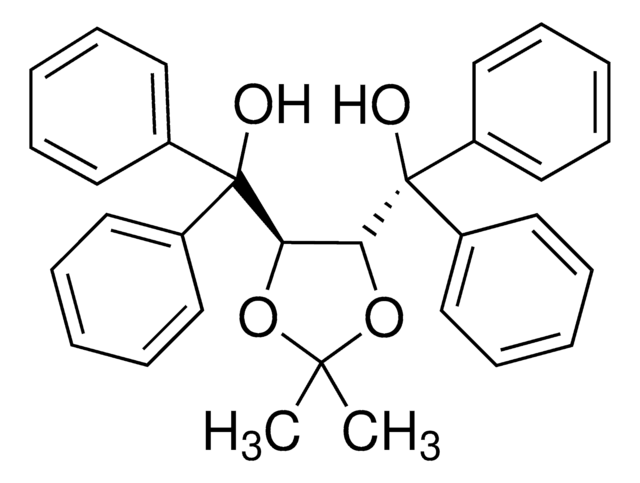
![Chlorocyclopentadienyl[(4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanolato]titanium 97%](/deepweb/assets/sigmaaldrich/product/structures/232/672/2ca86719-0965-4619-b25b-aae4087a2aad/640/2ca86719-0965-4619-b25b-aae4087a2aad.png)


![Zinc bis[bis(trimethylsilyl)amide] 97%](/deepweb/assets/sigmaaldrich/product/structures/294/819/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d/640/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d.png)