104655
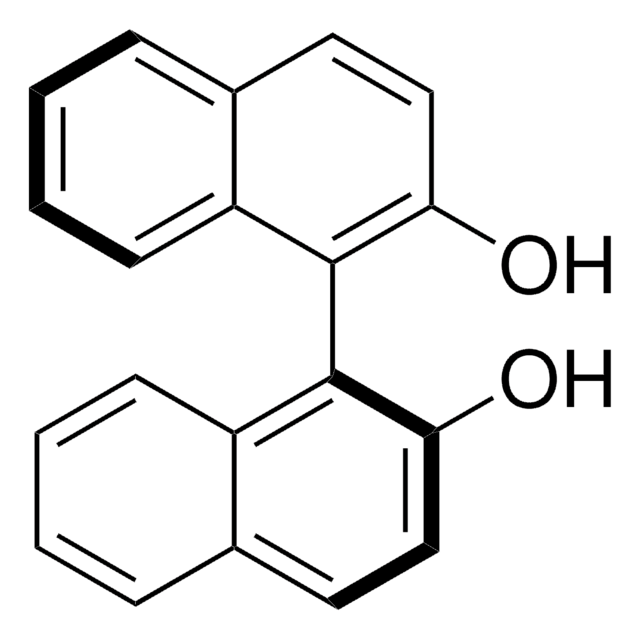
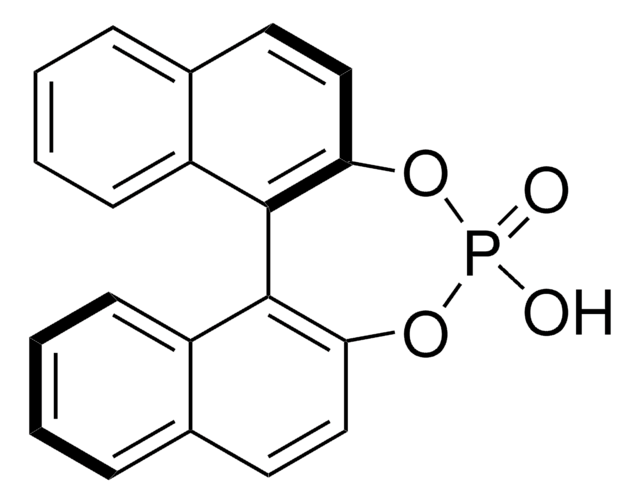
1,1′-Bi-2-naphthol
99%
동의어(들):
(±)-1,1′-Binaphthalene-2,2′-diol, (±)-2,2′-Dihydroxy-1,1′-dinaphthyl, 2,2′-Dihydroxybinaphthalene, 2,2′-Dihydroxydinaphthyl, 2,2′-Dinaphthol, BINOL
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
HOC10H6C10H6OH
CAS Number:
Molecular Weight:
286.32
Beilstein:
997518
EC Number:
MDL number:
UNSPSC 코드:
12161600
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
99%
반응 적합성
reagent type: ligand
mp
214-217 °C (lit.)
SMILES string
Oc1ccc2ccccc2c1-c3c(O)ccc4ccccc34
InChI
1S/C20H14O2/c21-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12,21-22H
InChI key
PPTXVXKCQZKFBN-UHFFFAOYSA-N
애플리케이션
Chiral ligand and auxiliary for asymmetric Michael addition reaction; enantioselective Diels-Alder reaction; alkynylation.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
BINOL: a versatile chiral reagent.
Jean Michel Brunel
Chemical reviews, 105(3), 857-897 (2005-03-10)
Yifeng Zhou et al.
Organic letters, 6(23), 4147-4149 (2004-11-05)
The readily available and inexpensive (S)-BINOL ligand in combination with Ti(O(i)Pr)(4) is an effective chiral catalyst for the catalytic asymmetric addition of alkynylzinc to unactivated simple ketones. Good to excellent enantioselectivities were achieved. No previous case has been reported successfully
Bixia Yao et al.
Journal of chromatography. A, 1216(28), 5429-5435 (2009-06-09)
In this work, the enantioseparations of 1,1'-bi-2-naphthol (BINOL) and its three derivatives were performed on an immobilized polysaccharide-based chiral stationary phase, Chiralpak IA, under normal-phase mode. The effects of the content of polar modifier in the mobile phase and the
Fengping Zhan et al.
Journal of chromatography. A, 1217(26), 4278-4284 (2010-05-15)
Enantioseparation of 1,1'-bi-2-naphthol (BINOL) was performed on a polysaccharide-based chiral stationary phase, Chiralcel OD-H, under normal-phase mode. The effects of polar modifier in the mobile phase on the retention, enantioseparation and elution order were investigated in detail. Solvent-induced reversal of
Na Ji et al.
Journal of the American Chemical Society, 128(27), 8845-8848 (2006-07-06)
Chiral sum-frequency (SF) spectroscopy that measures both the real and the imaginary components of the SF spectral response was demonstrated for the first time. It was based on interference of the SF signal with a dispersionless SF reference. Solutions of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.