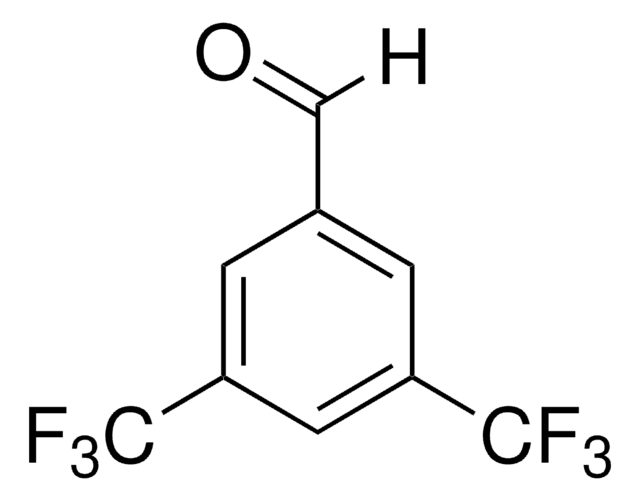
384038
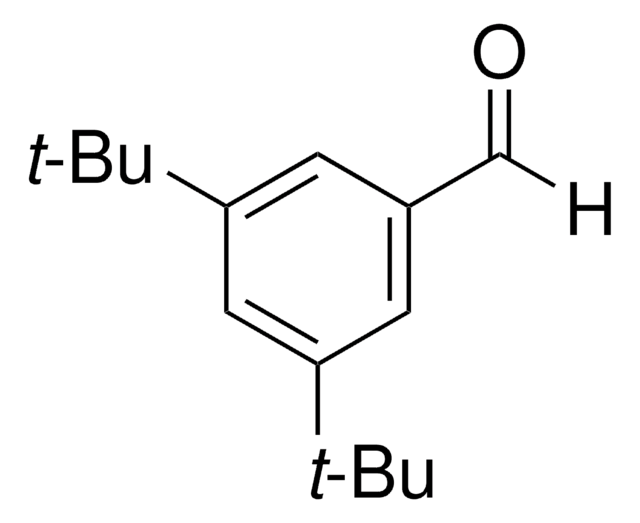
4-tert-Butylbenzaldehyde
97%
동의어(들):
4-(1,1-Dimethylethyl)benzaldehyde, p-tert-Butylbenzaldehyde, para-tert-Butylbenzaldehyde
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
(CH3)3CC6H4CHO
CAS Number:
Molecular Weight:
162.23
Beilstein:
1906461
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
양식
liquid
refractive index
n20/D 1.53 (lit.)
bp
130 °C/25 mmHg (lit.)
density
0.97 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)c1ccc(cc1)C(C)(C)C
InChI
1S/C11H14O/c1-11(2,3)10-6-4-9(8-12)5-7-10/h4-8H,1-3H3
InChI key
OTXINXDGSUFPNU-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
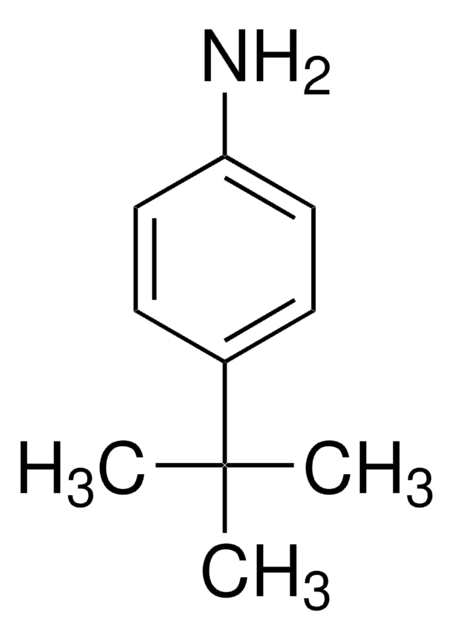
4-tert-Butylbenzaldehyde is an important intermediate for the synthesis of medicines, dyes, flavor and fragrance compounds. It is reported to be formed during the partial oxidation of 4-tert-butyltoluene by hydrogen peroxide in glacial acetic acid, catalyzed by bromide ions in combination with cobalt(II) acetate or cerium(III) acetate. Schiff base reaction between 4-tert-butylaniline and 4-tert-butylbenzaldehyde in ethanol has been carried out on-chip in the matrix assisted laser desorption ionization (MALDI) chamber, the formed imine was detected in real time.
애플리케이션
4-tert-Butylbenzaldehyde is suitable for use in a kinetic study to evaluate the kinetic constants (KI) for inhibition of mushroom tyrosinase by 4-substituted benzaldehydes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Repr. 2 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
213.8 °F - closed cup
Flash Point (°C)
101 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Leon G A van de Water et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(28), 8037-8044 (2007-07-12)
The partial oxidation of 4-tert-butyltoluene to 4-tert-butylbenzaldehyde by hydrogen peroxide in glacial acetic acid, catalyzed by bromide ions in combination with cobalt(II) acetate or cerium(III) acetate, has been studied in detail. Based on the observed differences in reaction rates and
Monica Brivio et al.
Lab on a chip, 5(4), 378-381 (2005-03-26)
The integration of a monitoring port along the microfluidic path of a MALDI-chip integrated device is described. Optimization of the microreactor design allows longer reaction and measuring times. The Schiff base reaction between 4-tert-butylaniline (1) and 4-tert-butylbenzaldehyde (2) in ethanol
M Jiménez et al.
Journal of agricultural and food chemistry, 49(8), 4060-4063 (2001-08-22)
A kinetic study of the inhibition of mushroom tyrosinase by 4-substituted benzaldehydes showed that these compounds behave as classical competitive inhibitors, inhibiting the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) by mushroom tyrosinase (o-diphenolase activity). The kinetic parameter (K(I)) characterizing this inhibition was
Chao-Bin Xue et al.
Bioorganic & medicinal chemistry, 15(5), 2006-2015 (2007-01-30)
Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-quinones. Inhibition of PO may provide a basis for novel environmentally friendly
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.