382000
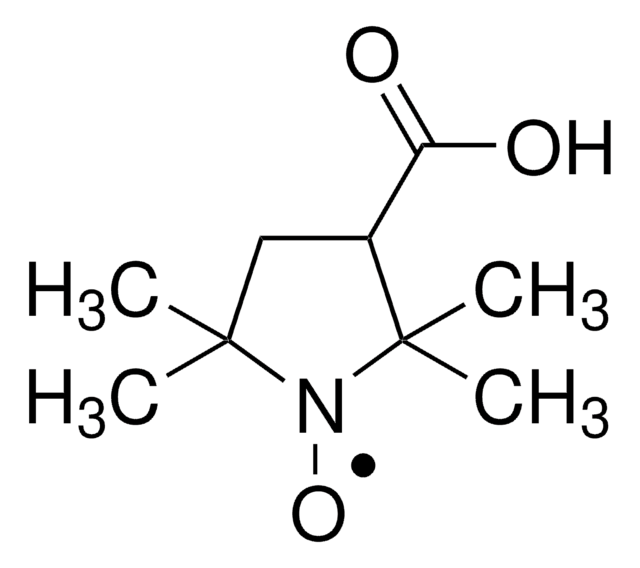
4-Carboxy-TEMPO, free radical
97%
동의어(들):
4-Carboxy-2,2,6,6-tetramethylpiperidine 1-oxyl, 4-Carboxy-2,2,6,6-tetramethylpiperidinyloxy, free radical, 4-Carboxy-TEMPO
About This Item
추천 제품
분석
97%
mp
185-189 °C (lit.)
작용기
carboxylic acid
저장 온도
2-8°C
SMILES string
CC1(C)CC(CC(C)(C)N1[O])C(O)=O
InChI
1S/C10H18NO3/c1-9(2)5-7(8(12)13)6-10(3,4)11(9)14/h7H,5-6H2,1-4H3,(H,12,13)
InChI key
CYQGCJQJIOARKD-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
문서
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.