모든 사진(3)
About This Item
Linear Formula:
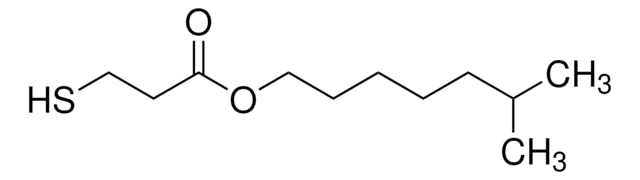
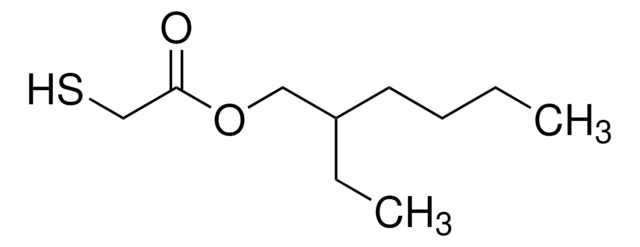
HSCH2CH2CO2(CH2)3CH3
CAS Number:
Molecular Weight:
162.25
EC Number:
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
추천 제품
Quality Level
분석
98%
refractive index
n20/D 1.457 (lit.)
bp
101 °C/12 mmHg (lit.)
density
0.999 g/mL at 25 °C (lit.)
SMILES string
CCCCOC(=O)CCS
InChI
1S/C7H14O2S/c1-2-3-5-9-7(8)4-6-10/h10H,2-6H2,1H3
InChI key
MGFFVSDRCRVHLC-UHFFFAOYSA-N
일반 설명
Butyl 3-mercaptopropionate (3MPA) is a monofunctional thiol that can be used as a cross-linker and chain transferring agent for controlling the molecular weight of the polymer. It can be used in the thiolene photopolymerization.
애플리케이션
3MPA can be used as a ligand which can functionalize the quantum dots for the development of high luminescence light emitting diodes. It can also be used as a crosslinking monomeric unit for the preparation of thiol-acrylate based photopolymers.
Used as a reactant for thiol-yne photopolymerizations to form highly-cross-linked networks.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
199.4 °F - closed cup
Flash Point (°C)
93 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Thiol- Yne photopolymerizations: novel mechanism, kinetics, and step-growth formation of highly cross-linked networks
Fairbanks BD, et al.
Macromolecules, 42(1), 211-217 (2008)
Development of a poly (methyl methacrylate-co-n-butyl methacrylate) copolymer binder system
Vail NK, et al.
Journal of Applied Polymer Science, 52(6), 789-812 (1994)
Benjamin D Fairbanks et al.
Macromolecules, 42(1), 211-217 (2009-05-23)
Radical-mediated thiol-yne step-growth photopolymerizations are utilized to form highly cross-linked polymer networks. This reaction mechanism is shown to be analogous to the thiol-ene photopolymerization; however, each alkyne functional group is capable of consecutive reaction with two thiol functional groups. The
High luminescence efficiency white light emitting diodes based on surface functionalized quantum dots dispersed in polymer matrices
Yoon C, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 428(22), 86-91 (2013)
Initiation and kinetics of thiol-ene photopolymerizations without photoinitiators
Cramer NB, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 42(22), 5817-5826 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)