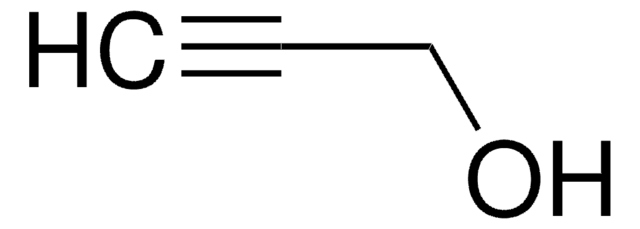추천 제품
Grade
technical grade
Quality Level
분석
90%
양식
liquid
refractive index
n20/D 1.459 (lit.)
bp
133-134 °C (lit.)
density
1.162 g/mL at 25 °C (lit.)
작용기
chloro
hydroxyl
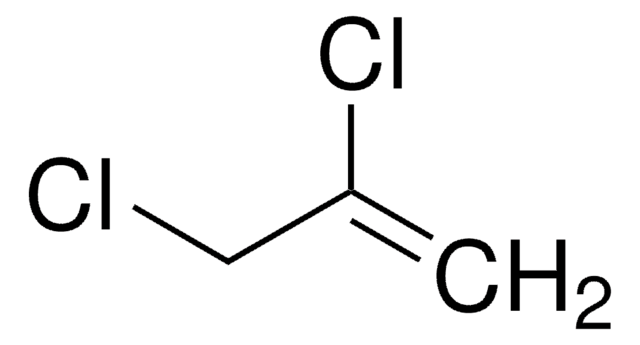
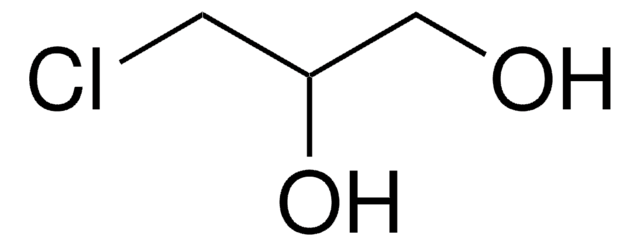
SMILES string
OCC(Cl)=C
InChI
1S/C3H5ClO/c1-3(4)2-5/h5H,1-2H2
InChI key
OSCXYTRISGREIM-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
2-Chloro-2-propen-1-ol is reported to undergo photodissociation at 193nm to generate CH2CCH2OH radical intermediate. 2-Chloro-2-propen-1-ol is formed as major product during base mediated reaction of 1,2,3-trichloropropane. 2-Chloro-2-propen-1-ol is reported to react with phosphorus trichloride to yield phosphorous esters, while with phosphory chloride it yields phosphoric ester.
애플리케이션
2-Chloro-2-propen-1-ol (2-chloropropenol) may be employed as carbon supplement for the growth of Pseudomonas strains. It may be used in the preparation of 2-(4-octylphenyl)prop-2-en-1-ol.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
129.2 °F - closed cup
Flash Point (°C)
54 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Kinetic studies of the homogeneous abiotic reactions of several chlorinated aliphatic compounds in aqueous solution.
Pagan M, et al.
Applied Geochemistry, 13(6), 779-785 (1998)
J J van der Waarde et al.
Applied and environmental microbiology, 59(2), 528-535 (1993-02-01)
Three Pseudomonas strains capable of utilizing 2-chloroallylalcohol (2-chloropropenol) as the sole carbon source for growth were isolated from soil. The fastest growth was observed with strain JD2, with a generation time of 3.6 h. Degradation of 2-chloroallylalcohol was accompanied by
Ran Zhu et al.
Journal of medicinal chemistry, 50(25), 6428-6435 (2007-11-13)
Compound 1 (FTY720, Fingolimod) represents a new generation of immunosuppressant that modulates lymphocyte trafficking by interacting with the S1P(1) receptor. Compound 1 also provides a template molecule for studying the molecular biology of S1P receptors and related enzymes (kinases and
Derivatives of phosphorus acids and 2-chloro-2-propen-1-ol.
Arbuzov BA, et al.
Russian Chemical Bulletin, 16(6), 1233-1238 (1967)
Arjun S Raman et al.
The Journal of chemical physics, 127(15), 154316-154316 (2007-10-24)
These velocity map imaging experiments characterize the photolytic generation of one of the two radical intermediates formed when OH reacts via an addition mechanism with allene. The CH2CCH2OH radical intermediate is generated photolytically from the photodissociation of 2-chloro-2-propen-1-ol at 193
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.