추천 제품
Quality Level
분석
98%
mp
103-105 °C (lit.)
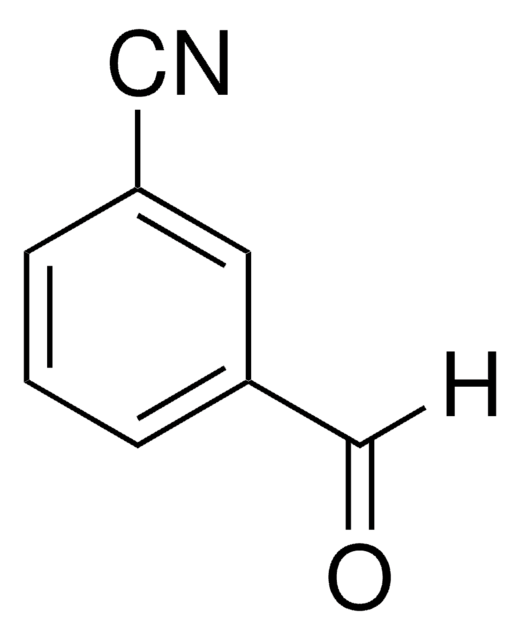
작용기
aldehyde
nitrile
SMILES string
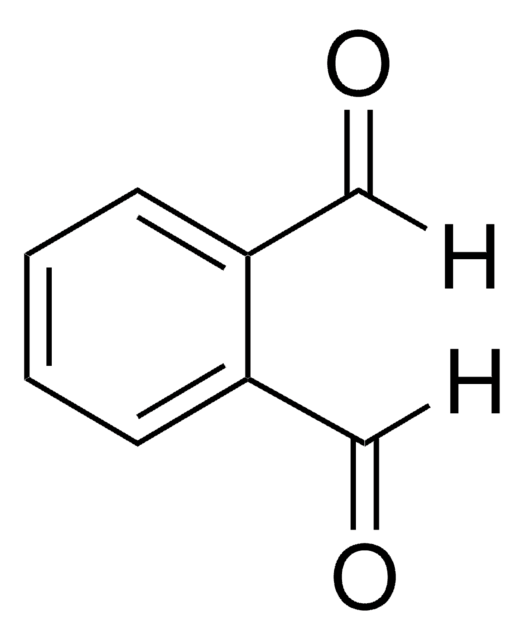
O=Cc1ccccc1C#N
InChI
1S/C8H5NO/c9-5-7-3-1-2-4-8(7)6-10/h1-4,6H
InChI key
QVTPWONEVZJCCS-UHFFFAOYSA-N
일반 설명
Aldol addition of enolizable 1,3-dicarbonyl compounds to 2-cyanobenzaldehyde in the presence of a tertiary amine has been reported. Biotransformation of 2-cyanobenzaldehyde by Euglena gracilis Z cultured photohetero-trophically has been reported.
애플리케이션
2-Cyanobenzaldehyde may be used:
- in the base-catalyzed one-pot synthesis of 3-substituted isoindolinones
- in the synthesis of 3-oxo-2,3-dihydro-1H-isoindoles, via Baylis-Hillman reaction
- in the synthesis of 3-(N-substituted amino)-1-isoindolenones
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Application of baylis-hillman methodology in a new synthesis of 3-oxo-2, 3-dihydro-1H-isoindoles.
Song YS, et al.
Journal of Heterocyclic Chemistry, 40(5), 939-942 (2003)
The aldol addition of readily enolizable 1, 3-dicarbonyl compounds to 2-cyanobenzaldehyde in the synthesis of novel 3-substituted isoindolinones.
More V, et al.
Synthesis, 2011(18), 3027-3031 (2011)
Biotransformation of aromatic aldehydes and related compounds by Euglena gracilis Z.
Noma Y, et al.
Phytochemistry, 30(9), 2969-2972 (1991)
Antonia Di Mola et al.
Beilstein journal of organic chemistry, 11, 2591-2599 (2016-01-07)
New bifunctional chiral ammonium salts were investigated in an asymmetric cascade synthesis of a key building block for a variety of biologically relevant isoindolinones. With this chiral compound in hand, the development of further transformations allowed for the synthesis of
Marcus Angelin et al.
The Journal of organic chemistry, 75(17), 5882-5887 (2010-08-10)
The mechanism of a base-catalyzed one-pot reaction of 2-cyanobenzaldehyde and primary nitroalkanes, to produce 3-substituted isoindolinones, has been investigated. A route starting with a nitroaldol (Henry) reaction, followed by a subsequent cyclization and rearrangement, was supported by intermediate analogue synthesis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.