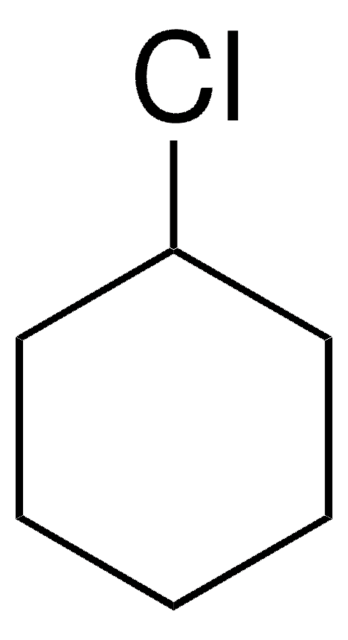
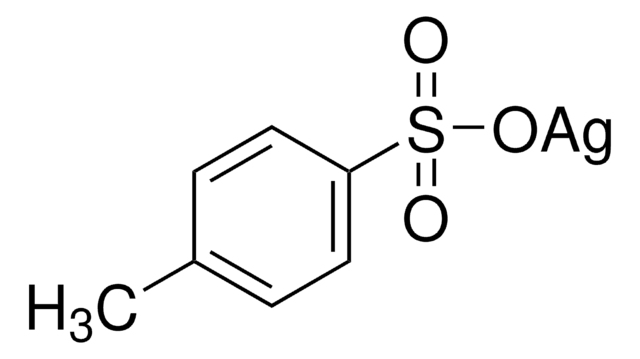
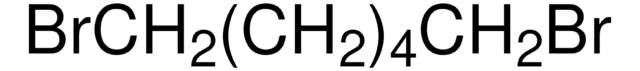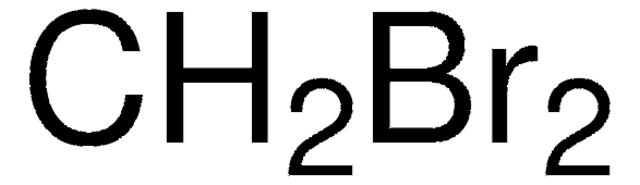모든 사진(1)
About This Item
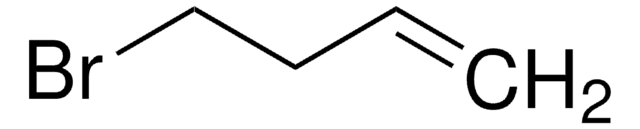
실험식(Hill 표기법):
C6H9Br
CAS Number:
Molecular Weight:
161.04
Beilstein:
635953
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
technical grade
분석
90%
양식
liquid
refractive index
n20/D 1.528 (lit.)
bp
57-58 °C/12 mmHg (lit.)
density
1.4 g/mL at 25 °C (lit.)
작용기
bromo
저장 온도
−20°C
SMILES string
BrC1CCCC=C1
InChI
1S/C6H9Br/c7-6-4-2-1-3-5-6/h2,4,6H,1,3,5H2
InChI key
AJKDUJRRWLQXHM-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
3-Bromocyclohexene was used in the synthesis of N-tert-butoxycarbonyl-O-cyclohexyl-L-tyrosine. It was also used in the synthesis of enantiopure cyclohexitols such as muco-quercitol, D-chiro-inocitol and allo-inocitol.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
129.2 °F - closed cup
Flash Point (°C)
54 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Y Nishiyama et al.
Chemical & pharmaceutical bulletin, 49(2), 233-235 (2001-02-24)
A facile and efficient synthesis of N-tert-butoxycarbonyl-O-cyclohexyl-L-tyrosine [Boc-Tyr(Chx)-OH] is described. Boc-Tyr-OH was treated with NaH in dimethylformamide and then with 3-bromocyclohexene to give N-Boc-O-(cyclohex-2-enyl)-L-tyrosine [Boc-Tyr(Che)-OH] in 70% yield. Hydrogenation of Boc-Tyr(Che)-OH over PtO2 afforded Boc-Tyr(Chx)-OH in almost quantitative yield. The
Synthesis of enantiopure cyclitols from (?)-3-bromocyclohexene mediated by intramolecular oxyselenenylation employing (S, S)-hydrobenzoin and (S)-mandelic acid as chiral sources.
Lee YJ, et al.
Tetrahedron, 61(8), 1987-2001 (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.