모든 사진(1)
About This Item
Linear Formula:
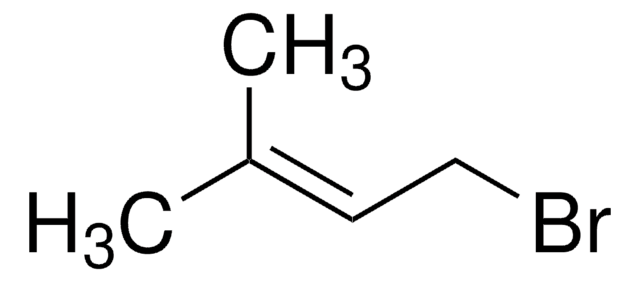
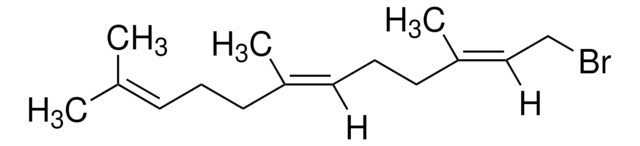
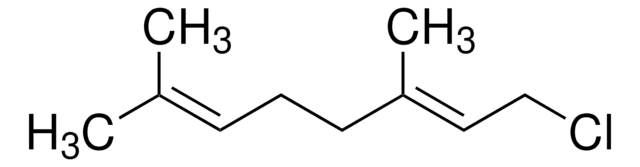
(CH3)2C=CHCH2CH2C(CH3)=CHCH2Br
CAS Number:
Molecular Weight:
217.15
Beilstein:
1703631
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
95%
형태
liquid
refractive index
n20/D 1.504 (lit.)
bp
101-102 °C/12 mmHg (lit.)
density
1.094 g/mL at 25 °C (lit.)
작용기
alkyl halide
저장 온도
2-8°C
SMILES string
C\C(C)=C\CC\C(C)=C\CBr
InChI
1S/C10H17Br/c1-9(2)5-4-6-10(3)7-8-11/h5,7H,4,6,8H2,1-3H3/b10-7+
InChI key
JSCUZAYKVZXKQE-JXMROGBWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Geranyl bromide undergoes palladium catalyzed cross-coupling reaction with aryl and alkenylgold(I) phosphanes.
애플리케이션
Geranyl bromide was used in synthesis of baicalein and 3,7-dihydroxyflavone derivatives. It was also used in synthesis of potential flavonoidic modulators of P-glycoprotein activity.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
167.0 °F - closed cup
Flash Point (°C)
75 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
M Maitrejean et al.
Bioorganic & medicinal chemistry letters, 10(2), 157-160 (2000-02-15)
A new series of potential flavonoidic modulators of P-glycoprotein activity has been prepared. The flavanolignan silybin was first oxidised to dehydrosilybin and then C-alkylated with either prenyl or geranyl bromide. The resulting isoprenoid dehydrosilybins were shown to display high in
Marta Perro Neves et al.
European journal of medicinal chemistry, 46(6), 2562-2574 (2011-04-19)
Fourteen baicalein and 3,7-dihydroxyflavone derivatives were synthesized and evaluated for their inhibitory activity against the in vitro growth of three human tumor cell lines. The synthetic approaches were based on the reaction with prenyl or geranyl bromide in alkaline medium
Palladium-catalyzed cross-coupling reactions of organogold(I) phosphanes with allylic electrophiles.
Miguel Peña-López et al.
Organic & biomolecular chemistry, 10(8), 1686-1694 (2012-01-24)
Aryl and alkenylgold(I) phosphanes react regioselectively with allylic electrophiles such as cinnamyl and geranyl halides (bromide, chloride and acetates) under palladium catalysis in THF at 80 °C to afford the α-substitution product with moderate to high yields. When the reaction
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.