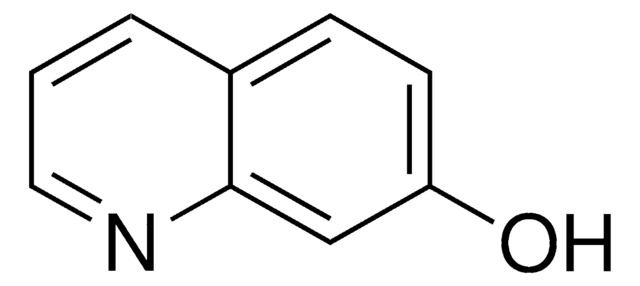
추천 제품
분석
95%
mp
188-190 °C (lit.)
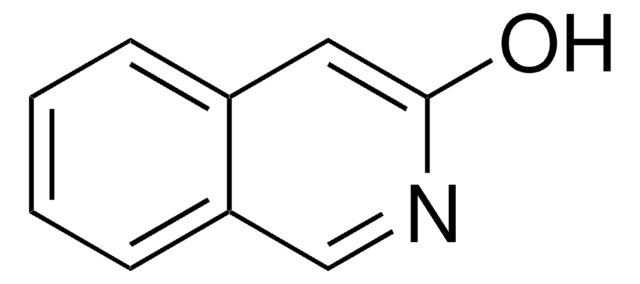
SMILES string
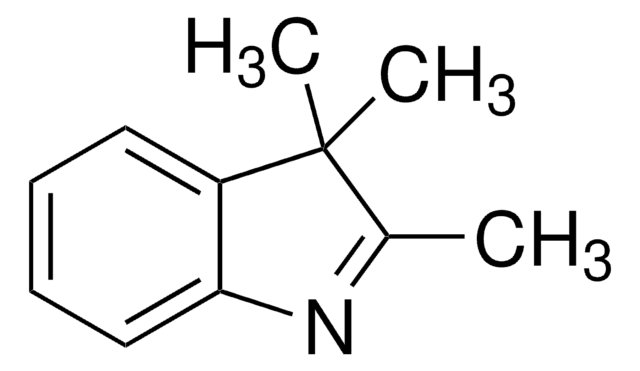
Oc1ccc2ncccc2c1
InChI
1S/C9H7NO/c11-8-3-4-9-7(6-8)2-1-5-10-9/h1-6,11H
InChI key
OVYWMEWYEJLIER-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
6-Hydroxyquinoline is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. The excited-state proton transfer and geminate recombination of 6-hydroxyquinoline encaged in catalytic Na+-exchanged faujasite zeolites X and Y have been explored by measuring steady-state and picosecond time-resolved spectra.
애플리케이션
6-Hydroxyquinoline was used in synthesis of 2,6-substituted-benzo[d]thiazole analogs and 2,4-substituted-benzo[d]thiazole analogs.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Katharigatta Narayanaswamy Venugopala et al.
European journal of medicinal chemistry, 65, 295-303 (2013-06-04)
A novel and efficient one pot synthesis was developed for 2,6-substituted-benzo[d]thiazole analogues 4a-k and 2,4-substituted-benzo[d]thiazole analogues 4l-pvia three component condensation reaction of substituted arylaldehyde, 2-amino-6-halo/4-methyl-benzo[d]thiazole and 2-naphthol or 6-hydroxyquinoline in presence of 10% w/v NaCl in water by microwave method.
Yu-Hui Liu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 128, 280-284 (2014-04-01)
6-Hydroxyquinoline (6HQ) is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. We have previously (Mahata et al. (2002)) shown that the ESPT reaction between 6HQ and trimethylamine (TMA) leads to an "unusual" emission in the 440-450 nm
Mohan Singh Mehata
The journal of physical chemistry. B, 112(28), 8383-8386 (2008-06-20)
A hydrogen-bonded network formed between 6-hydroxyquinoline (6-HQ) and acetic acid (AcOH) has been characterized using a time-resolved fluorescence technique. In the bridged hydrogen-bonded complex of cis-6-HQ and AcOH, an excited-state reaction proceeds via proton transfer along the hydrogen bond, resulting
Yu-Hui Liu et al.
The journal of physical chemistry. A, 115(1), 19-24 (2010-12-15)
Spectroscopic studies on excited-state proton transfer (ESPT) of hydroxyquinoline (6HQ) have been performed in a previous paper. And a hydrogen-bonded network formed between 6HQ and acetic acid (AcOH) in nonpolar solvents has been characterized. In this work, a time-dependent density
Junhong Qian et al.
Physical chemistry chemical physics : PCCP, 12(39), 12562-12569 (2010-08-21)
Photophysical properties of the organocatalyst cupreidine (CPD) and its chromophoric building block 6-hydroxyquinoline (6HQ) in protic and nonprotic polar solvents (methanol and acetonitrile) were investigated by means of UV-vis absorption, and steady state and time resolved fluorescence spectroscopy. The effects
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.