301019
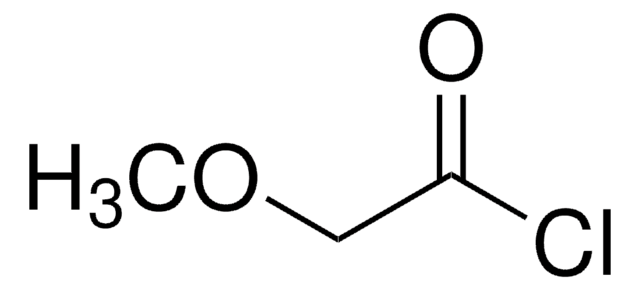
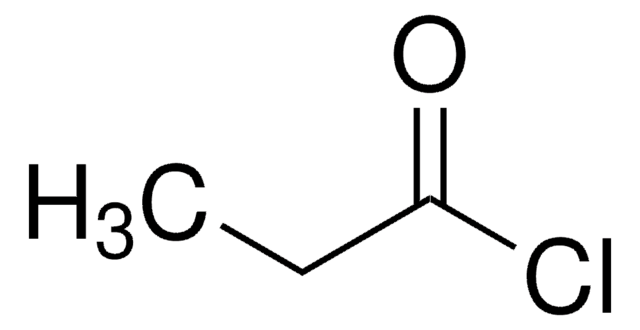
Benzyloxyacetyl chloride
95%
동의어(들):
α-(Benzyloxy)acetyl chloride, (Phenylmethoxy)acetyl chloride, 2-(Benzyloxy)acetyl chloride, 2-(Phenylmethoxy)acetyl chloride, 2-Phenylmethoxyacetyl chloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C6H5CH2OCH2COCl
CAS Number:
Molecular Weight:
184.62
Beilstein:
1947363
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
양식
liquid
refractive index
n20/D 1.523 (lit.)
bp
84-87 °C/0.4 mmHg (lit.)
density
1.17 g/mL at 25 °C (lit.)
작용기
acyl chloride
ether
phenyl
저장 온도
2-8°C
SMILES string
ClC(=O)COCc1ccccc1
InChI
1S/C9H9ClO2/c10-9(11)7-12-6-8-4-2-1-3-5-8/h1-5H,6-7H2
InChI key
QISAUDWTBBNJIR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Benzyloxyacetyl chloride was used in preparation of:
- (S)-3-(methylamino)-3-((R)-pyrrolidin-3-yl)propanenitrile, key intermediate in the preparation of fluoroquinolone antibiotic for respiratory tract infections
- non-racemic helicene
- β-lactams
- substituted 2-azetidinones for further elaboration into annulated β-lactams
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
보충제 위험성
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
The Journal of Organic Chemistry, 58, 1646-1646 (1993)
Manjinder S Lall et al.
The Journal of organic chemistry, 77(10), 4732-4739 (2012-04-25)
(S)-3-(methylamino)-3-((R)-pyrrolidin-3-yl)propanenitrile (1) is a key intermediate in the preparation of PF-00951966, (1) a fluoroquinolone antibiotic for use against key pathogens causing community-acquired respiratory tract infections including multidrug resistant (MDR) organisms. The current work describes the development of a highly efficient
Synthesis, structure, and properties of a helical columnar liquid crystal.
Nuckolls C and Katz TJ.
Journal of the American Chemical Society, 120(37), 9541-9544 (1998)
Willem Van Brabandt et al.
The Journal of organic chemistry, 71(18), 7083-7086 (2006-08-26)
A high-yielding, asymmetric synthesis of novel 4-formyl-1-(2- and 3-haloalkyl)azetidin-2-ones was developed as valuable starting materials for the synthesis of different enantiomerically enriched bicyclic azetidin-2-ones, such as piperazine, morpholine, and 1,4-diazepane annulated beta-lactam derivatives. Especially the hydride reduction of 4-imidoyl-1-(2- and
The Journal of Organic Chemistry, 59, 932-932 (1994)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.