287636
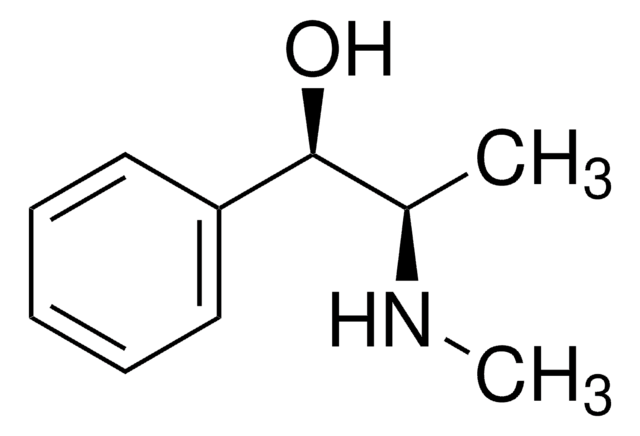
(1S,2S)-(+)-Pseudoephedrine
98%
동의어(들):
(+)-Pseudoephedrine, (+)-ψ-Ephedrine, (1S,2S)-2-Methylamino-1-phenyl-1-propanol, d-Isoephedrine, d-Pseudoephedrine
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
실험식(Hill 표기법):
C10H15NO
CAS Number:
Molecular Weight:
165.23
Beilstein:
2414132
EC Number:
MDL number:
UNSPSC 코드:
12352116
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
(1S,2S)-(+)-Pseudoephedrine, a natural enantiomer of ephedrine, is a decongestant commonly used in cold and allergy medicines.
애플리케이션
(1S,2S)-(+)-Pseudoephedrine condenses with N,N-diisopropyl-2-formyl-1-naphthamide to form the corresponding oxazolidine derivative as a single diasterisomer.
(1S,2S)-(+)-Pseudoephedrine may be used as a chiral auxillary in asymmetric synthesis of enantioenriched organic compounds. It may also be used to prepare a novel tertiary pseudo C2-symmetric 1,2-diamine, which facilitates the enantioselective addition of methyl lithium to imines with better yield.
생화학적/생리학적 작용
Non-selective adrenergic agonist; decongestant
애플리케이션
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
James K Cunningham et al.
Drug and alcohol dependence, 126(1-2), 55-64 (2012-05-18)
Clandestine laboratory operators commonly extract ephedrine and pseudoephedrine-precursor chemicals used to synthesize methamphetamine-from over-the-counter cold/allergy/sinus products. To prevent this activity, two states, Oregon in 07/2006 and Mississippi in 07/2010, implemented regulations classifying ephedrine and pseudoephedrine as Schedule III substances, making
Beatriz Alonso et al.
The Journal of organic chemistry, 78(2), 614-627 (2012-12-25)
We have developed an efficient protocol for carrying out the stereocontrolled formal conjugate addition of hydroxycarbonyl anion equivalents to α,β-unsaturated carboxylic acid derivatives using (S,S)-(+)-pseudoephedrine as chiral auxiliary, making use of the synthetic equivalence between the heteroaryl moieties and the
(-)-Ephedrine as an auxiliary for the asymmetric synthesis of atropisomeric amides by dynamic resolution under thermodynamic control.
Clayden J and Lai LW.
Tetrahedron Letters, 42(18), 3163-3166 (2001)
A new pseudo C2-symmetric tertiary diamine for the enantioselective addition of MeLi to aromatic imines.
Gille S, et al.
Tetrahedron Asymmetry, 17(7), 1045-1047 (2006)
Pseudoephenamine: a practical chiral auxiliary for asymmetric synthesis.
Marvin R Morales et al.
Angewandte Chemie (International ed. in English), 51(19), 4568-4571 (2012-03-31)
Chromatograms
application for HPLCapplication for HPLCapplication for HPLC자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.