추천 제품
분석
97%
mp
237-239 °C (lit.)
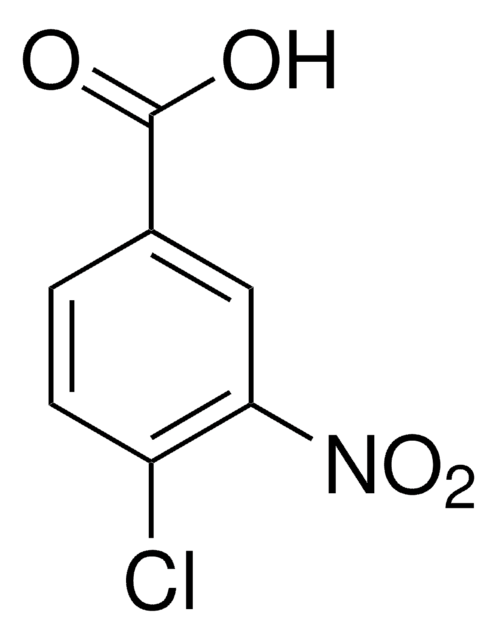
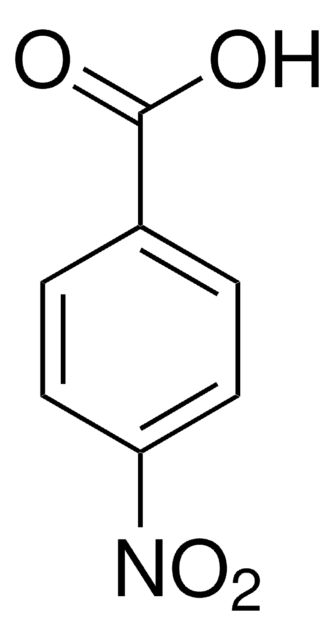
SMILES string
OC(=O)c1cccc(Cl)c1[N+]([O-])=O
InChI
1S/C7H4ClNO4/c8-5-3-1-2-4(7(10)11)6(5)9(12)13/h1-3H,(H,10,11)
InChI key
VCHSXYHBMFKRBK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Hydrogen-bonded structures of isomeric compounds of 3-chloro-2-nitrobenzoic acid with quinoline have been investigated.
애플리케이션
3-Chloro-2-nitrobenzoic acid has been used in the preparation of:
- 2-amino-3-chlorobenzonitrile
- 3-chloro-2-nitrobenzaldehyde
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Tadashi Kataoka et al.
Bioorganic & medicinal chemistry, 12(9), 2397-2407 (2004-04-15)
Condensation of nitrobenzaldehydes 3 and alpha-[o-(p-methoxybenzylthio)benzoyl] sulfoxide 4 gave alpha-sulfinyl enones 5. Treatment of 5 with formic acid caused cyclization followed by debenzylation to afford 3-(methylsulfinyl)thioflavanones 6. Double-bond formation with elimination of methanesulfenic acid was performed by refluxing 6 in
Kazuma Gotoh et al.
Acta crystallographica. Section C, Crystal structure communications, 65(Pt 10), o534-o538 (2009-10-07)
The structures of four isomeric compounds, all C7H4ClNO4.C9H7N, of quinoline with chloro- and nitro-substituted benzoic acid, namely, 2-chloro-5-nitrobenzoic acid-quinoline (1/1), (I), 3-chloro-2-nitrobenzoic acid-quinoline (1/1), (II), 4-chloro-2-nitrobenzoic acid-quinoline (1/1), (III), and 5-chloro-2-nitrobenzoic acid-quinoline (1/1), (IV), have been determined at 185 K.
The synthesis and in vitro acetylcholinesterase and butyrylcholinesterase inhibitory activity of tacrine (Cognex?) derivaties.
Gregor VE, et al.
Bioorganic & Medicinal Chemistry Letters, 2(8), 861-864 (1992)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.