270822
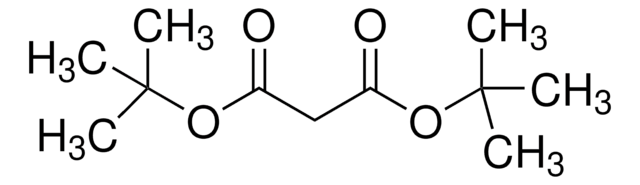
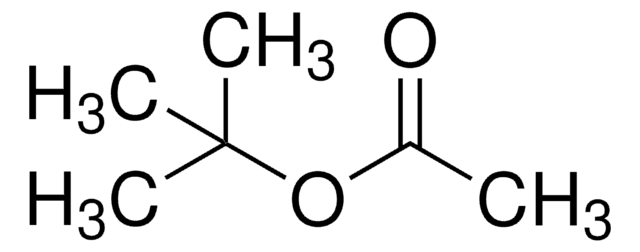
Di-tert-butyl acetylenedicarboxylate
98%
동의어(들):
2-Butynedioic acid di-tert-butyl ester, Di-tert-butyl 2-butynedioate
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
(CH3)3COCOC≡CCOOC(CH3)3
CAS Number:
Molecular Weight:
226.27
Beilstein:
1957547
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
The cross-cyclotrimerization of di-tert-butyl acetylenedicarboxylate, silylacetylenes and acrylamides was studied with cationic rhodium(I)/(R)-tol-binap complex as catalyst. Glycosyl azides were subjected to 1,3-dipolar cycloaddition with di-tert-butyl acetylenedicarboxylate.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
W Bröder et al.
Carbohydrate research, 249(1), 221-241 (1993-10-18)
Glycosyl azides provide reliable anomeric protection stable to conditions for hydrolytic removal of ester groups, for reductive opening or release of acetalic diol protection, for the introduction of ether-type protection, and for glycosylation processes. The utility of this anomeric protection
Jun Hara et al.
Angewandte Chemie (International ed. in English), 53(11), 2956-2959 (2014-02-08)
It has been established that a cationic rhodium(I)/(R)-tol-binap complex catalyzes the cross-cyclotrimerization of silylacetylenes, di-tert-butyl acetylenedicarboxylates, and acrylamides with excellent chemo-, regio-, and enantioselectivities. Unsymmetrical alkynoates can also be employed in place of di-tert-butyl acetylenedicarboxylate for this process, but with
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.