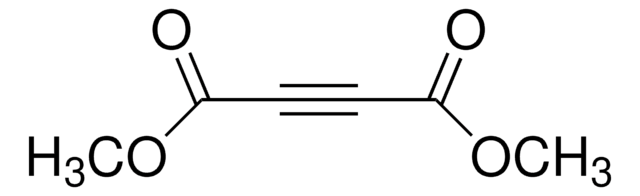
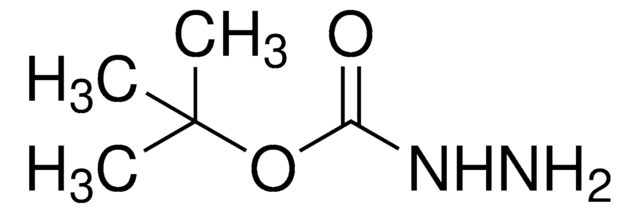
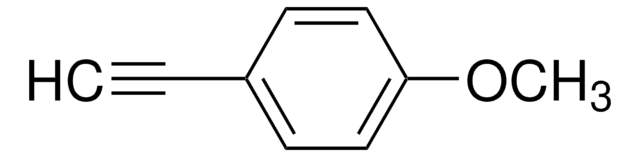
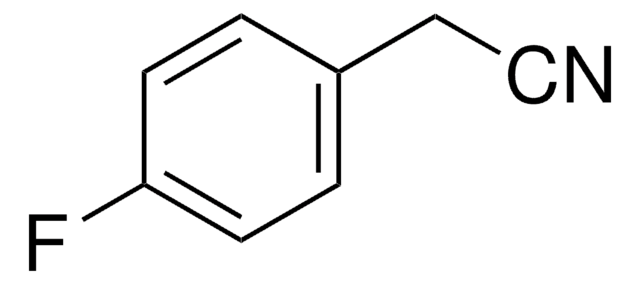
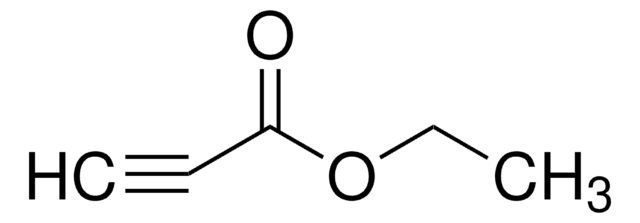
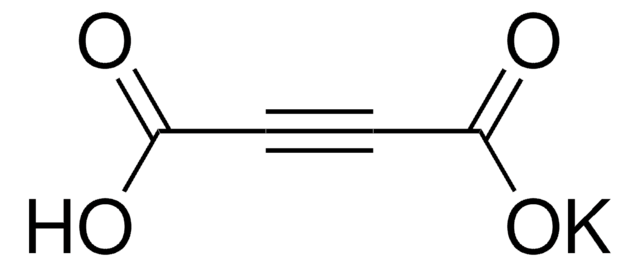추천 제품
일반 설명
Diethyl acetylenedicarboxylate is a protein cross-linker.
Diethyl acetylenedicarboxylate is used as a Michael acceptor for O-vinyl oximes synthesis, and is used in the nucleophilic addition reaction.
Diethyl acetylenedicarboxylate is used as a Michael acceptor for O-vinyl oximes synthesis, and is used in the nucleophilic addition reaction.
애플리케이션
Diethyl acetylenedicarboxylate was used in the synthesis of:
- 3,4,5-trisubstituted 2(5H)-furanone derivatives
- highly functionalized thiazolidinone derivatives
- novel cyclic peroxide glucosides
- 4,11-dimesitylbisanthene, soluble bisanthene derivative, via Diels-Alder reaction
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
201.2 °F - closed cup
Flash Point (°C)
94 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Eric H Fort et al.
Journal of the American Chemical Society, 131(44), 16006-16007 (2009-10-17)
A soluble bisanthene derivative, 4,11-dimesitylbisanthene, has been synthesized in three steps from bianthrone. In hot toluene, this bisanthene undergoes a clean Diels-Alder reaction with diethyl acetylenedicarboxylate to give a rearomatized 1:1 cycloadduct and, more slowly, a rearomatized 2:1 cycloadduct. In
W M Basyouni et al.
Drug research, 65(9), 473-478 (2014-09-11)
A series of 3,4,5-trisubstituted 2(5H)-furanone derivatives was synthesized through one-pot reaction of amines, aldehydes and diethyl acetylenedicarboxylate. Silica sulfuric acid efficiently catalyzes the 3-component reaction to afford the corresponding 2(5H)-furanones in high yields. The synthesized compounds were tested against HEPG2
Di-Zao Li et al.
Journal of Asian natural products research, 11(7), 613-620 (2010-02-26)
Four novel cyclic peroxide glucosides 15a, 15b, 16a, and 16b, optically pure analogs of shuangkangsu (1), which is an anti-virus natural product with an unusual skeleton isolated from the buds of Lonicera japonica Thunb, were first synthesized totally in six
Kh Mahid Uddin et al.
Dalton transactions (Cambridge, England : 2003), 46(39), 13597-13609 (2017-09-28)
The reactivity of the face-capped benzothiazolate clusters HOs
Abdelmadjid Benmohammed et al.
Molecules (Basel, Switzerland), 19(3), 3068-3083 (2014-03-13)
We present herein the synthesis in good yields of two series of highly functionalized thiazolidinone derivatives from the reactions of various 4-phenyl-3-thio-semicarbazones with ethyl 2-bromoacetate and diethyl acetylenedicarboxylate, respectively.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.