추천 제품
애플리케이션
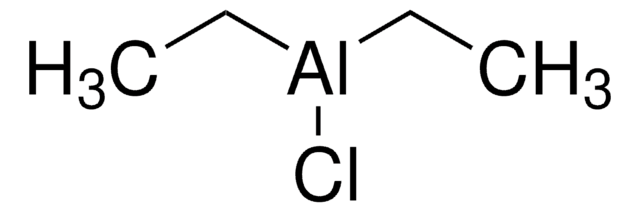
Ethylaluminum dichloride is a strong Lewis acid and a proton scavenger that can be used:
- To catalyze polymerization of isobutylene.
- To facilitate Diels-Alder reaction of α,β-unsaturated esters.
- To carry out Pinacol reduction rearrangements.
- To promote Schmidt rearrangement of diketoazides in the synthesis of indolizidines and pyrroloazepinediones.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 1 Inhalation - STOT SE 3 - Water-react 2
표적 기관
Central nervous system, Nervous system
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point (°F)
-9.4 °F - closed cup
Flash Point (°C)
-23 °C - closed cup
이미 열람한 고객
Concise access to indolizidine and pyrroloazepine skeleta via intramolecular Schmidt reactions of azido 1, 3-diketones.
Lertpibulpanya D and Marsden S P
Organic & Biomolecular Chemistry, 4(18), 3498-3504 (2006)
Cyclorearrangement and cycloolefination of keto-bis-sulfones. A sulfone analog of a pinacol reduction-rearrangement.
Trost B M, et al.
Journal of the American Chemical Society, 114(13), 5432-5434 (1992)
Kinetic and Mechanistic Studies of the Polymerization of Isobutylene Catalyzed by EtAlCl2/Bis (2-chloroethyl) Ether Complex in Hexanes.
Banerjee S, et al.
Macromolecules, 48(16), 5474-5480 (2015)
Ethylaluminum Dichloride.
Snider B B.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Ethylaluminum Dichloride.
Snider B B.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2001)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.