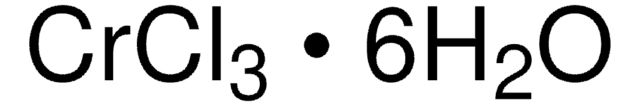추천 제품
vapor density
4.4 (vs air)
Quality Level
vapor pressure
150 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
≥99%
양식
liquid
반응 적합성
reagent type: oxidant
불순물
<10 ppb Heavy metals
색상
APHA: 0-150
refractive index
n20/D 1.429 (lit.)
bp
62-65 °C (lit.)
mp
−10-−8 °C (lit.)
density
1.5 g/mL at 20 °C (lit.)
작용기
acyl chloride
SMILES string
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
InChI key
CTSLXHKWHWQRSH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Oxalyl chloride is a commonly used chlorinating reagent that can be prepared by the reaction of oxalic acid and phosphorus pentachloride.
애플리케이션
Oxalyl chloride may be used in the following processes:
- Preparation of Mosher′s acid chloride by reacting with Mosher′s acid in the presence of DMF.
- Activation of dimethyl sulfoxide for use in the oxidation of long-chain alcohols to carbonyls.
- Activation of α-keto carboxylic acids and N-heterocyclic carboxylic acids for alkynylation to form ynediones and N-heterocyclic ynones, respectively.
Reactant involved in:
- Synthesis of N-heterocyclic ynones and ynediones, used to activate carboxylic acids
- Chlorination and halogenation
- Three-component [3+2] cycloadditions
- Reactions with organostannanes
- Synthesis of cyclopentenones
- Carbonylations, used as a carbonyl synthon
Suitable for the synthesis of acid chlorides used to produce liquid crystals.
포장
The 5g, 25g and 100g units sold in the US are packaged in ampules.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Water-react 1
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point (°F)
51.8 °F - closed cup
Flash Point (°C)
11.0 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles
이미 열람한 고객
A simple method for the microscale preparation of Mosher's acid chloride.
Ward DE and Rhee CK.
Tetrahedron Letters, 32(49), 7165-7166 (1991)
Catalytic Syntheses of N-Heterocyclic Ynones and Ynediones by In Situ Activation of Carboxylic Acids with Oxalyl Chloride.
Boersch C, et al.
Angewandte Chemie (International Edition in English), 50(44), 10448-10452 (2011)
Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide" activated" by oxalyl chloride.
Mancuso AJ, et al.
The Journal of Organic Chemistry, 43(12), 2480-2482 (1978)
Benoît Heurtaux et al.
The Journal of organic chemistry, 70(4), 1474-1477 (2005-02-12)
[reaction: see text] Several natural pulvinic acids were synthesized. Silyl ketene acetals derived from methyl arylacetates (4 equiv) reacted with oxalyl chloride at -78 degrees C, without the need of adding a catalyst. After treatment of the crude diketones with
Peter J Manley et al.
Organic letters, 4(18), 3127-3129 (2002-08-31)
[reaction: see text] A mild, practical, one-pot method for the generation of imidoyl chlorides and their subsequent in situ reaction with pyridine-1-oxides is described. The imidoyl chlorides were formed from the reaction of secondary amides with a stoichiometric amount of
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 221015-100G | 4061838776846 |
| 221015-10KG | 4061838161192 |
| 221015-25G | 4061838776853 |
| 221015-5G | 4061838776860 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.