모든 사진(2)
About This Item
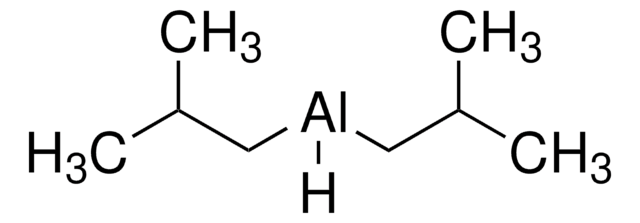
Linear Formula:
[(CH3)2CHCH2]2AlH
CAS Number:
Molecular Weight:
142.22
Beilstein:
4123663
MDL number:
UNSPSC 코드:
12352001
eCl@ss:
38120609
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Diisobutylaluminum hydride solution (1M in THF) is a powerful reducing agent. It can be used in the following reactions:
- Synthesis of trans-alkene isosteres of protected dipeptides.
- To generate bis(1,5-cyclooctadiene)nickel(0) (Ni(cod)2) in situ, which can catalyze the conjugate addition of ethenyltributyltin to 2-propenal to form tert-butyldimethyl[((E)-1,4pentadienyl)oxy]silane.
- Reduction of the arylpropiolate esters to give the corresponding propargyl alcohol.
Used in Pd-catalyzed reductive debromination of secondary alkyl bromides. O-Debenzylation and ring opening of perbenzylated furanosides. Convenient in situ generation of HZrCp2Cl from ZrCp2Cl2 and DIBAL-H.
포장
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles
이미 열람한 고객
A stereocontrolled synthesis of trans-alkene isosteres of dipeptides.
Spaltenstein A, et al.
Tetrahedron Letters, 27(19), 2095-2098 (1986)
Damien Webb et al.
Organic letters, 14(2), 568-571 (2011-12-31)
A continuous flow system for the multiparameter (flow rate, temperature, residence time, stoichiometry) optimization of the DIBALH reduction of esters to aldehydes is described. Incorporating an in-line quench (MeOH), these transformations are generally complete in fewer than 60 s. Mixing
Hidetsura Cho et al.
The Journal of organic chemistry, 75(3), 627-636 (2009-12-31)
A systematic investigation of the reductive ring-expansion reaction of cyclic ketoximes fused to aromatic rings with diisobutylaluminum hydride (DIBALH) is described. This reaction regioselectively afforded a variety of five- to eight-membered bicyclic heterocycles or tricyclic heterocycles containing nitrogen neighboring an
D J Kopecky et al.
The Journal of organic chemistry, 65(1), 191-198 (2000-05-18)
An optimized protocol for the DIBALH reductive acetylation of acyclic esters and diesters is described. This reductive acetylation procedure allows a wide variety of esters to be converted into the corresponding alpha-acetoxy ethers in good to excellent yields. It was
J Marco-Contelles et al.
Carbohydrate research, 335(1), 63-70 (2001-09-13)
The reaction of DIBALH with bis(heteroannulated)-pyranosides containing the perhydrofuro[2,3-b]pyran moiety is described. The hydride attack at the anomeric carbon (C-9a) resulted in the exclusive tetrahydrofuran ring opening. The selectivity of this reaction has been evaluated as other benzylidene acetals built
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.