모든 사진(1)
About This Item
실험식(Hill 표기법):
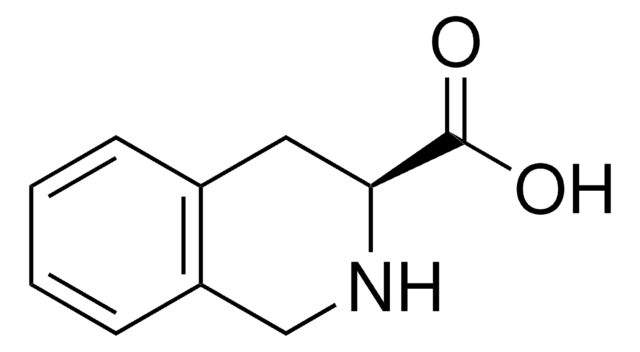
C10H11NO2 · HCl
CAS Number:
Molecular Weight:
213.66
Beilstein:
3723332
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
96%
mp
>300 °C (lit.)
SMILES string
Cl[H].OC(=O)C1Cc2ccccc2CN1
InChI
1S/C10H11NO2.ClH/c12-10(13)9-5-7-3-1-2-4-8(7)6-11-9;/h1-4,9,11H,5-6H2,(H,12,13);1H
InChI key
FXHCFPUEIDRTMR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid was used in the synthesis of 10,10a-dihydroimidazo-[1,5-b]isoquinoline-1,3(2H,5H)-diones, inhibitor of inflammation, apoprotein B-100 biosynthesis and matrix-degrading metalloprotienase.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Alan R Katritzky et al.
The Journal of organic chemistry, 67(23), 8224-8229 (2002-11-09)
Condensations of chiral diamines 11a-c with benzotriazole and formaldehyde gave benzotriazolyl intermediates 12a-c; similar condensations of alpha-amino-amides 10a-c with benzotriazole and paraformaldehyde gave 14a-c. Subsequent treatment of 12a-c and 14a-c with AlCl(3) led to enantiopure tricyclic 1,2,3,5,10,10a-hexahydroimidazo[1,5-b]isoquinolines 1a-c and 2,3,10,10a-tetrahydroimidazo[1,5-b]isoquinolin-1(5H)-ones
Raman K Bakshi et al.
Bioorganic & medicinal chemistry letters, 15(14), 3430-3433 (2005-06-14)
The discovery of 1-amino-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid analogs as potent human melanocortin-4 selective agonists is described.
B C Wilkes et al.
Biopolymers, 34(9), 1213-1219 (1994-09-01)
A molecular mechanics study (grid search and energy minimization) of the highly delta receptor-selective delta opioid antagonist H-Tyr-Tic-Phe-OH (TIP; Tic: tetrahydroisoquinoline-3-carboxylic acid) resulted in four low energy conformers with energies within 2 kcal/mol of that of the lowest energy structure.
M Manning et al.
Journal of peptide science : an official publication of the European Peptide Society, 1(1), 66-79 (1995-01-01)
We have investigated the effects of mono-substitutions with the conformationally restricted amino acid, 1,2,3,4 tetrahydroisoquinoline-3-carboxylic acid (Tic) at position 3 in arginine vasopressin (AVP), at positions 2, 3 and 7 in potent non-selective cyclic AVP V2/V1a antagonists, in potent and
Yingjie Zhang et al.
Current protein & peptide science, 11(8), 752-758 (2011-01-18)
Tic, short for 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, is a kind of unnatural α-amino acids. Due to its distinct geometrical conformation and biological activity, the structure of Tic, regarded as the surrogate of proline and the rigid analogue of phenylalanine or tyrosine, has
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![1,3-Bis[4-(dimethylamino)phenyl]-2,4-dihydroxycyclobutenediylium dihydroxide, bis(inner salt) Dye content 90 %](/deepweb/assets/sigmaaldrich/product/structures/301/519/500149b3-198c-44cf-b952-7e91f54fc48e/640/500149b3-198c-44cf-b952-7e91f54fc48e.png)
![2,4-Bis[4-(N,N-diphenylamino)-2,6-dihydroxyphenyl]squaraine 98%](/deepweb/assets/sigmaaldrich/product/structures/303/054/d8b9c845-3623-4f5a-8a30-ab6731034171/640/d8b9c845-3623-4f5a-8a30-ab6731034171.png)