모든 사진(1)
About This Item
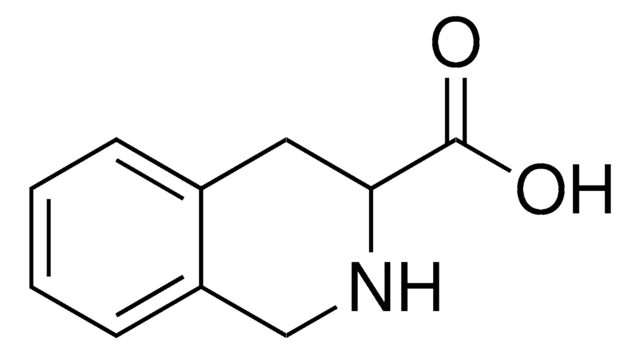
실험식(Hill 표기법):
C10H11NO2
CAS Number:
Molecular Weight:
177.20
Beilstein:
4842199
MDL number:
UNSPSC 코드:
12352106
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
형태
solid
광학 활성
[α]20/D −168°, c = 1.8 in 1.4 M NaOH
반응 적합성
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
응용 분야
peptide synthesis
SMILES string
OC(=O)[C@@H]1Cc2ccccc2CN1
InChI
1S/C10H11NO2/c12-10(13)9-5-7-3-1-2-4-8(7)6-11-9/h1-4,9,11H,5-6H2,(H,12,13)/t9-/m0/s1
InChI key
BWKMGYQJPOAASG-VIFPVBQESA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Raman K Bakshi et al.
Bioorganic & medicinal chemistry letters, 15(14), 3430-3433 (2005-06-14)
The discovery of 1-amino-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid analogs as potent human melanocortin-4 selective agonists is described.
B C Wilkes et al.
Biopolymers, 34(9), 1213-1219 (1994-09-01)
A molecular mechanics study (grid search and energy minimization) of the highly delta receptor-selective delta opioid antagonist H-Tyr-Tic-Phe-OH (TIP; Tic: tetrahydroisoquinoline-3-carboxylic acid) resulted in four low energy conformers with energies within 2 kcal/mol of that of the lowest energy structure.
M Manning et al.
Journal of peptide science : an official publication of the European Peptide Society, 1(1), 66-79 (1995-01-01)
We have investigated the effects of mono-substitutions with the conformationally restricted amino acid, 1,2,3,4 tetrahydroisoquinoline-3-carboxylic acid (Tic) at position 3 in arginine vasopressin (AVP), at positions 2, 3 and 7 in potent non-selective cyclic AVP V2/V1a antagonists, in potent and
Yingjie Zhang et al.
Current protein & peptide science, 11(8), 752-758 (2011-01-18)
Tic, short for 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, is a kind of unnatural α-amino acids. Due to its distinct geometrical conformation and biological activity, the structure of Tic, regarded as the surrogate of proline and the rigid analogue of phenylalanine or tyrosine, has
P Majer et al.
International journal of peptide and protein research, 43(1), 62-68 (1994-01-01)
A new method of synthesizing ortho-methylated phenylalanines has been developed. Phenylalanines with at least one free ortho-position undergo a Pictet-Spengler cyclization with formaldehyde followed by hydrogenolytic splitting of the endocyclic benzylic C--N bond of 1,2,3,4-tetrahydroisoquinolines and afford corresponding ortho-methyl derivatives.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.