추천 제품
분석
99%
mp
90-92 °C (lit.)
solubility
water: soluble 50 mg/mL, clear, colorless to faintly yellow
density
1.611 g/mL at 25 °C (lit.)
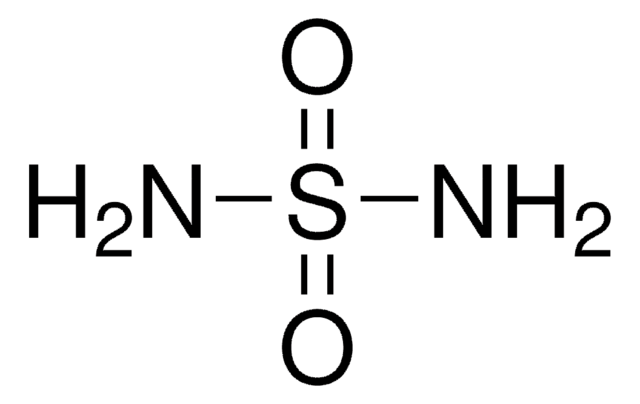
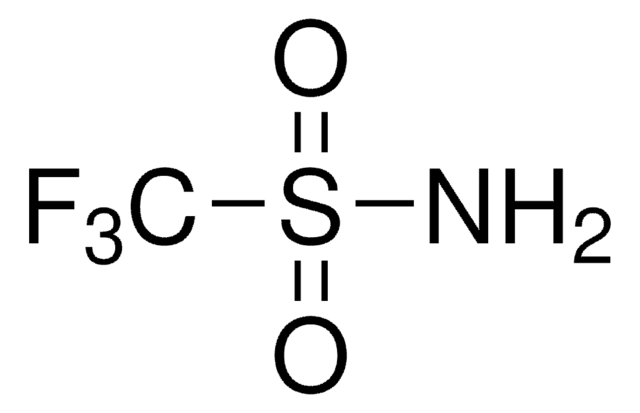
SMILES string
NS(N)(=O)=O
InChI
1S/H4N2O2S/c1-5(2,3)4/h(H4,1,2,3,4)
InChI key
NVBFHJWHLNUMCV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Sulfamide, a polar aprotic solvent compatible with Grignard reagents, is used as a functional group in medicinal chemistry.
애플리케이션
Sulfamide was used in the synthesis of:
- Schiff bases of the type ArCH=NSO2NH2
- 1H,3H-2,1,3-benzothiadiazin-4-one-2,2-dioxide (BTDD)
- sulfamide analogs of oleoylethanolamide analogs in a study of PPARα activation.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Anna Di Fiore et al.
Bioorganic & medicinal chemistry letters, 20(12), 3601-3605 (2010-05-18)
We investigated the inhibition of carbonic anhydrase (CA, EC 4.2.1.1) isoforms I-XV with 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenylsulfamide and other simple or sugar sulfamides, a class of less investigated CA inhibitors (CAIs). The crystal structure of the adduct of hCA II with the boron-substituted
A Scozzafava et al.
Journal of enzyme inhibition, 15(5), 443-453 (2000-10-13)
Sulfamide and sulfamic acid are the simplest compounds containing the SO2NH2 moiety, responsible for binding to the Zn(II) ion within carbonic anhydrase (CA, EC 4.2.1.1) active site, and thus acting as inhibitors of the many CA isozymes presently known. Here
Carolina Cano et al.
Journal of medicinal chemistry, 50(2), 389-393 (2007-01-19)
Long chain saturated and unsaturated alkyl sulfamide and propyl sulfamide derivatives, analogs of oleoylethanolamide, have been synthesized and evaluated in vivo and in vitro as peroxisome proliferator activated receptor alpha (PPARalpha) activators. Additionally, the anorexic effects of the new compounds
Bacterial cleavage of nitrogen to sulfone bonds in sulfamide and 1H-2, 1, 3-benzothiadiazin-4 (3H)-one 2, 2-dioxide: formation of 2-nitrobenzamide by Gordonia sp.
Rein U and Cook AM.
FEMS Microbiology Letters, 172(2), 107-113 (1999)
Alessio Innocenti et al.
Bioorganic & medicinal chemistry letters, 19(4), 1155-1158 (2009-01-09)
The membrane-associated mouse isozyme of carbonic anhydrase XV (mCA XV), has been investigated for its interaction with anion inhibitors. mCA XV is an isoforms possessing a very particular inhibition profile by anions, dissimilar to that of all other mammalian CAs
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.