추천 제품
분석
99%
양식
liquid
refractive index
n20/D 1.593 (lit.)
bp
215 °C (lit.)
mp
−13 °C (lit.)
density
1.1322 g/mL at 20 °C (lit.)
작용기
fluoro
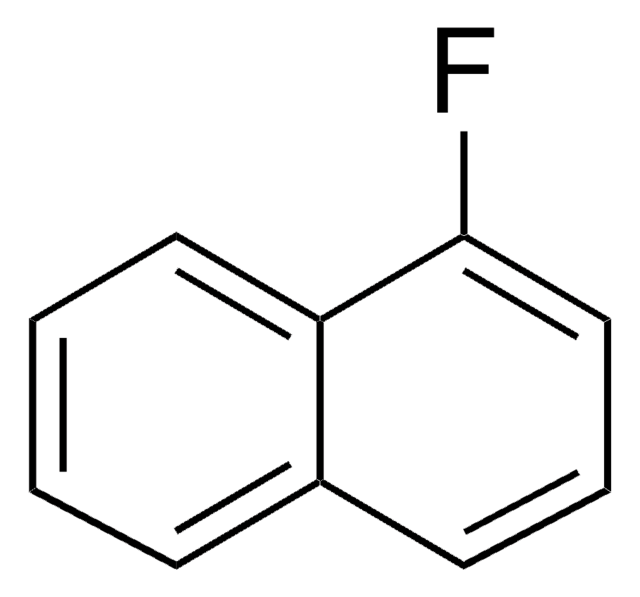
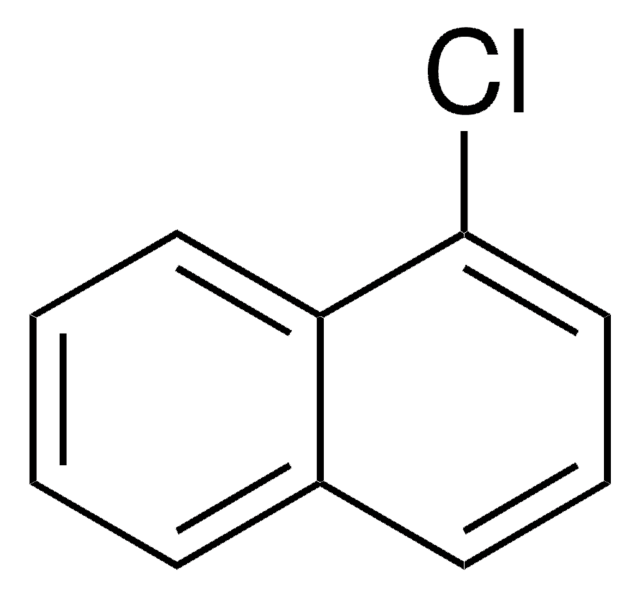
SMILES string
Fc1cccc2ccccc12
InChI
1S/C10H7F/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-7H
InChI key
CWLKTJOTWITYSI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Flash photolysis of O2 and 1-fluoronaphthalene mixtures in the gas phase have been investigated.
애플리케이션
1-Fluoronaphthalene was used in t-BuLi-mediated synthesis of 6-substituted phenanthridines. It was also used in the synthesis of LY248686, a potent inhibitor of serotonin and norepinephrine uptake.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
149.0 °F - closed cup
Flash Point (°C)
65 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Asymmetric synthesis and absolute stereochemistry of LY248686.
Deeter J, et al.
Tetrahedron Letters, 31(49), 7101-7104 (1990)
Jan Pawlas et al.
Organic letters, 4(16), 2687-2690 (2002-08-03)
[reaction: see text] A one-pot, t-BuLi-induced synthesis of 6-substituted phenanthridines from fluoroarenes and nitriles via 1,2-arynes is reported. Aryl- and hetaryl nitriles, cyanamides, and trimethylacetonitrile gave phenanthridine products. The method was extended to provide bisphenanthridine 10 by a one-pot bis-cyclization
Formation of O 2 (1Sigma g+) by 1-fluoronaphthalene sensitization.
Andrews LJ and Abrahamson EW.
Chemical Physics Letters, 10, 113-116 (1971)
C E Cerniglia et al.
Applied and environmental microbiology, 48(2), 294-300 (1984-08-01)
The metabolism of 1-fluoronaphthalene by Cunninghamella elegans ATCC 36112 was studied. The metabolites were isolated by reverse-phase high-pressure liquid chromatography and characterized by the application of UV absorption, 1H nuclear magnetic resonance, and mass spectral techniques. C. elegans oxidized 1-fluoronaphthalene
Peter Wipf et al.
Organic letters, 5(7), 1155-1158 (2003-03-28)
[reaction: see text] Electron-rich dinaphthyl ethers were synthesized by S(N)Ar reactions between naphthols and activated fluoronaphthalenes. 2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton's base) was found to be an excellent, mild alternative to traditional inorganic bases for promoting the coupling reaction.
프로토콜
US EPA Method 610 describes the analysis of polynuclear aromatic hydrocarbons (commonly referred to as PAHs or PNAs) by both HPLC and GC.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.