추천 제품
양식
liquid
Quality Level
반응 적합성
reagent type: reductant
농도
1.0 M in hexanes
density
0.675 g/mL at 25 °C
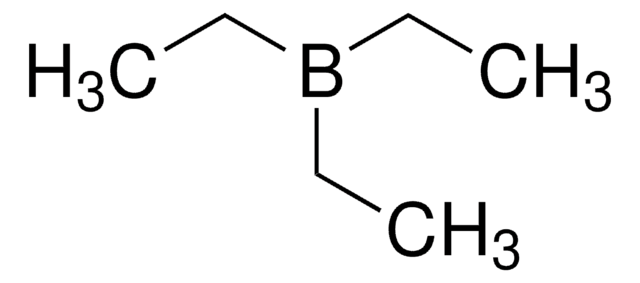
SMILES string
CCB(CC)CC
InChI
1S/C6H15B/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI key
LALRXNPLTWZJIJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Catalyst for:
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
- Allylation of aldehydes
- Decarboxylative C-C bond cleavage reactions
- Rhenium hydride / boron Lewis acid cocatalysis of alkene hydrogenations
- Regioselective hydroxyalkylation of unsaturated oxime ethers
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
Triethylborane can be used:
- As a radical initiator and terminator of free-radical reactions in aqueous media.(1)
- To synthesize polymers such as poly(2-substituted-1-propenylene)s by reacting with 2-substituted allylic arsonium ylides.(2)
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1A - STOT RE 1 Inhalation - STOT SE 3
표적 기관
Central nervous system, Nervous system
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point (°F)
-32.8 °F
Flash Point (°C)
-36 °C
Synthesis of poly (2-substituted-1-propenylene)s from allylic arsonium ylides.
Mondiere R, et al.
Macromolecules, 38(3), 663-668 (2005)
Free-radical reaction of imine derivatives in water.
Miyabe H, et al.
The Journal of Organic Chemistry, 65(16), 5043-5047 (2000)
Hideto Miyabe et al.
Chemical & pharmaceutical bulletin, 51(5), 540-544 (2003-05-09)
Stereocontrol in radical reactions of oxime ether anchored to polymer support was studied. Highly diastereoselective solid-phase radical reaction was achieved by using triethylborane and diethylzinc as a radical initiator at low reaction temperature, providing a novel method for the synthesis
Masafumi Ueda
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 124(6), 311-319 (2004-06-01)
The aqueous medium radical reactions of a variety of imine derivatives such as oxime ether, oxime, hydrazone, nitrone, and N-sulfonylimine were investigated. Triethylborane-mediated intermolecular alkyl radical addition to glyoxylic oxime ether, oxime, and nitrone in water proceeded smoothly to give
Ken-ichi Yamada et al.
The Journal of organic chemistry, 77(3), 1547-1553 (2012-01-03)
Triethylborane-mediated tin-free radical alkylation of N-alkoxycarbonyl-imines, such as N-Boc-, N-Cbz-, and N-Teoc-imines, proceeded smoothly at a low temperature (-78 to -20 °C) to give the corresponding adducts in high yield. Although the formation of isocyanate was the major unfavorable reaction
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.