추천 제품
Quality Level
분석
97%
refractive index
n20/D 1.588 (lit.)
bp
147-148 °C/3.5 mmHg (lit.)
density
1.045 g/mL at 25 °C (lit.)
작용기
hydroxyl
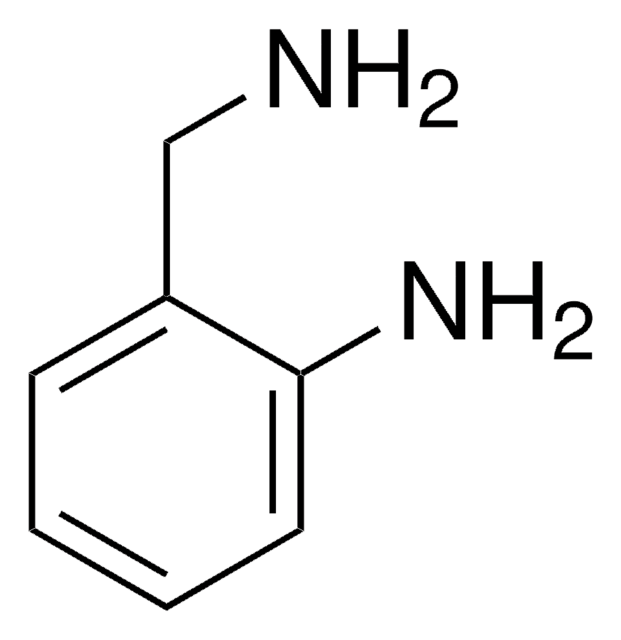
SMILES string
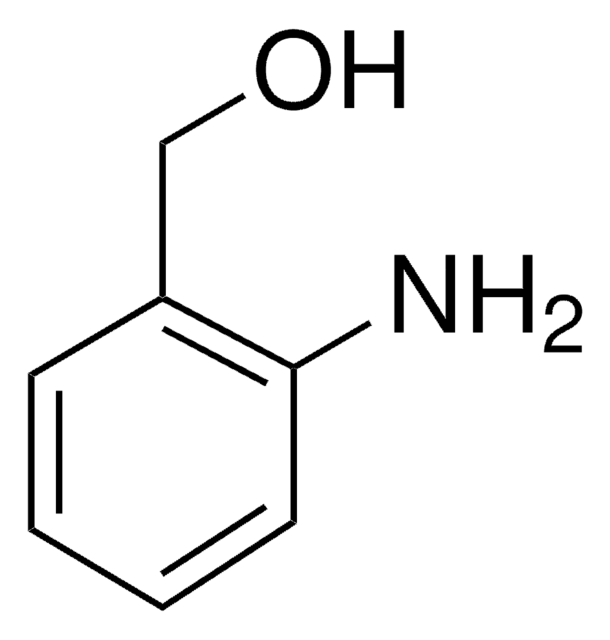
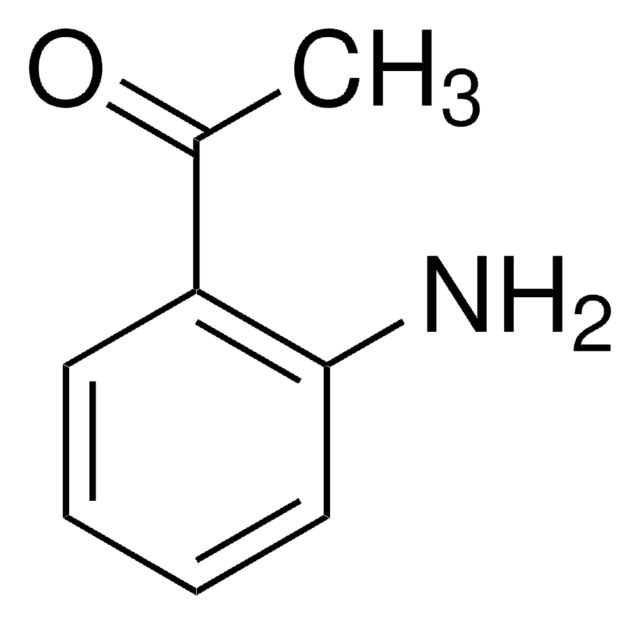
Nc1ccccc1CCO
InChI
1S/C8H11NO/c9-8-4-2-1-3-7(8)5-6-10/h1-4,10H,5-6,9H2
InChI key
ILDXSRFKXABMHH-UHFFFAOYSA-N
일반 설명
2-Aminophenethyl alcohol undergoes one-pot cyclization with carboxylic acids in the presence of PPh3, CCl4 and NEt3 to yield N-acyl indolines.
애플리케이션
2-Aminophenethyl alcohol was used in the synthesis of:
- indole derivatives
- N-(cyanothioformyl)indoline
- dihydro-3,1-benzoxazepine
기타 정보
This material may darken over time with minimal impact to chemical purity.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Zengxue Wang et al.
The Journal of organic chemistry, 72(24), 9364-9367 (2007-11-02)
A unique one-pot cyclization of 2-aminophenethyl alcohols with carboxylic acids in the presence of PPh3, CCl4, and NEt3 furnished the formation of N-acyl indolines in good to excellent yields. This new approach provides an efficient, scalable, low-cost, and direct access
N-(Cyanothioformyl) indoline; a new indoline ring forming reaction.
Besson T, et al.
Journal of the Chemical Society. Perkin Transactions 1, 24, 4057-4060 (1998)
Ruthenium-catalyzed dehydrogenative N-heterocyclization. Indoles from 2-aminophenethyl alcohols and 2-nitrophenethyl alcohols.
Tsuji Y, et al.
The Journal of Organic Chemistry, 55(2), 580-584 (1990)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.