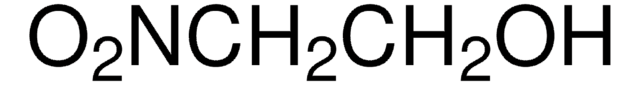192333
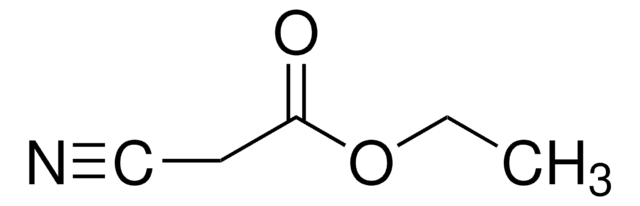
Ethyl nitroacetate
97%
동의어(들):
2-Nitroacetic acid ethyl ester, Ethyl 2-nitroacetate, Nitroacetic acid ethyl ester
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
NO2CH2CO2C2H5
CAS Number:
Molecular Weight:
133.10
Beilstein:
1210027
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
refractive index
n20/D 1.424 (lit.)
bp
105-107 °C/25 mmHg (lit.)
density
1.199 g/mL at 25 °C (lit.)
작용기
amine
ester
nitro
SMILES string
CCOC(=O)C[N+]([O-])=O
InChI
1S/C4H7NO4/c1-2-9-4(6)3-5(7)8/h2-3H2,1H3
InChI key
FTKASJMIPSSXBP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
Ethyl nitroacetate has been used in:
- synthesis of γ-oxoacids via Michael addition reaction with α,β-unsaturated ketones
- fuctionalization of C4-position on pyrimidine and C6-position on 2′-deoxyguanosine to produce novel nucleosides
- facile synthesis of α,α-diisobutylglycine
- synthesis of DL-4,4-difluoroglutamic acid
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point (°F)
197.6 °F - closed cup
Flash Point (°C)
92 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
T Tsukamoto et al.
Journal of medicinal chemistry, 39(1), 66-72 (1996-01-05)
DL-4,4-Difluoroglutamic acid (DL-4,4-F2Glu) and its methotrexate analogue, DL-gamma,gamma-difluoromethotrexate (DL-gamma,gamma-F2MTX), were synthesized and evaluated as alternate substrates or inhibitors of folate-dependent enzymes. Synthesis of DL-4,4-F2Glu involved the nitroaldol reaction of ethyl nitroacetate with a difluorinated aldehyde ethyl hemiacetal as a key
Indranil Bhattacharjee et al.
Physical chemistry chemical physics : PCCP, 20(9), 6060-6072 (2017-12-23)
Achieving synthetic control over light-driven molecular dynamics is essential for designing complex molecule-based devices. Here we design a novel coumarin-imidazole conjugate (1) whose excited state structural dynamics are primarily controlled by a distant intramolecular H-bonding interaction within the backbone. The
Elena Trogu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(7), 2081-2093 (2012-01-12)
Base-catalysed condensation reactions of nitroacetic esters with dipolarophiles to give isoxazole derivatives proceed faster, and often with higher yields, in the presence of water than in organic solvents such as chloroform. Kinetic profiles show that induction times are greatly reduced
Maialen Aginagalde et al.
The Journal of organic chemistry, 75(21), 7435-7438 (2010-10-05)
Michael addition of ethyl nitroacetate on α,β-unsaturated ketones followed by Nef oxidation under hydrolytic conditions yields γ-oxoacids instead of the corresponding α,δ-dioxoesters. A concerted decarboxylation step is proposed on the basis of computational results. Finally, conversion of the γ-ketoacids thus
Victor Timoshchuk
Nucleosides, nucleotides & nucleic acids, 24(5-7), 1043-1046 (2005-10-27)
A study of C-nucleophilic substitution at the C4-position on pyrimidine and C6-position on 2'-deoxyguanosine to produce novel nucleosides is presented with the spectroscopic properties of their respective substitution products. C4-(1,2,4-triazol-1-yl) pyrimidine nucleosides 1 were treated with nitroalkanes, malononitrile, acetylacetone, ethyl
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.