180769
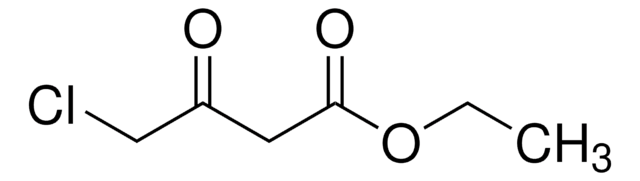
Ethyl 4-chloroacetoacetate
95%
동의어(들):
4-Chloro acetoethylacetate, Ethyl 4-chloro-3-oxobutanoate, Ethyl chloroacetoacetate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
ClCH2COCH2CO2C2H5
CAS Number:
Molecular Weight:
164.59
Beilstein:
1761275
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
양식
liquid
refractive index
n20/D 1.452 (lit.)
bp
115 °C/14 mmHg (lit.)
density
1.218 g/mL at 25 °C (lit.)
작용기
chloro
ester
ketone
SMILES string
CCOC(=O)CC(=O)CCl
InChI
1S/C6H9ClO3/c1-2-10-6(9)3-5(8)4-7/h2-4H2,1H3
InChI key
OHLRLMWUFVDREV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Ethyl 4-chloroacetoacetate was used in the synthesis of phosphorus ylides.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
206.6 °F - closed cup
Flash Point (°C)
97 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
H Yamamoto et al.
Applied microbiology and biotechnology, 67(1), 33-39 (2004-09-01)
Formate dehydrogenases (FDH) are useful for the regeneration of NADH, which is required for asymmetric reduction by several dehydrogenases and reductases. FDHs have relatively low activity and are labile, especially to alpha-haloketones, thus FDH cannot be applied to the industrial
Zhinan Xu et al.
Applied microbiology and biotechnology, 70(1), 40-46 (2005-09-22)
Escherichia coli M15 (pQE30-car0210) was constructed to express carbonyl reductase (CAR) by cloning the car gene from Candida magnoliae and inserting it into pQE30. By cultivating E. coli M15 (pQE30-car0210) and M15 (pQE30-gdh0310), 8.2-fold and 12.3-fold enhancements in specific enzymatic
Hou Cao et al.
Bioresource technology, 102(2), 1733-1739 (2010-10-12)
A novel NADH-dependent dehydrogenases/reductases (SDRs) superfamily reductase (PsCRII) was isolated from Pichia stipitis. It produced ethyl (S)-4-chloro-3-hydroxybutanoate [(S)-CHBE] in greater than 99% enantiomeric excess. This enzyme was purified to homogeneity by ammonium sulfate precipitation followed by Q-Sepharose chromatography. Compared to
Y Saratani et al.
Bioscience, biotechnology, and biochemistry, 65(7), 1676-1679 (2001-08-23)
The enantioselectivity of ECAA to ECHB by eight fungi of four genus was evaluated. All strains showed (S)-selectivity, and Cylindrocarpon sclerotigenum IFO 31855 gave the highest yield and good optical purity (e.e.; >99%). Cell-free extract and acetone-dried cells of C.
M Kataoka et al.
Applied microbiology and biotechnology, 51(4), 486-490 (1999-05-26)
The asymmetric reduction of ethyl 4-chloro-3-oxobutanoate (COBE) to ethyl (R)-4-chloro-3-hydroxybutanoate [(R)-CHBE] using Escherichia coli cells, which coexpress both the aldehyde reductase gene from Sporobolomyces salmonicolor and the glucose dehydrogenase (GDH) gene from Bacillus megaterium as a catalyst was investigated. In
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.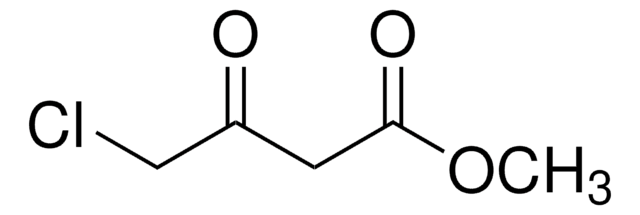

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)