추천 제품
Quality Level
분석
98%
bp
275-276 °C (lit.)
mp
59-61 °C (lit.)
SMILES string
Oc1ccc2CCCCc2c1
InChI
1S/C10H12O/c11-10-6-5-8-3-1-2-4-9(8)7-10/h5-7,11H,1-4H2
InChI key
UMKXSOXZAXIOPJ-UHFFFAOYSA-N
애플리케이션
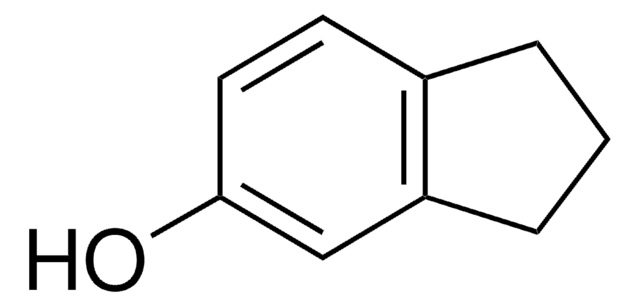
5,6,7,8-Tetrahydro-2-naphthol was used as a model compound in the study of photochemical transformation of 17β-estradiol (natural estrogenic steroid) and 17α-ethinylestradiol (synthetic oral contraceptive).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
T Bhattacharya et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 59(3), 525-535 (2003-01-14)
Both steady state and time resolved spectroscopic measurements reveal that the prime process involved in quenching mechanism of the lowest excited singlet (S1) and triplet (T1) states of the well known electron acceptor 9-Cyanoanthracene (9CNA) in presence of 5,6,7,8-tetrahydro-1-naphthol (TH1N)
B Kalyanaraman et al.
The Journal of biological chemistry, 259(22), 14018-14022 (1984-11-25)
Electron spin resonance spectroscopy has been used to demonstrate production of semiquinone-free radicals from the oxidation of the catechol estrogens 2- and 4-hydroxyestradiol and 2,6- and 4,6-dihydroxyestradiol. Radicals were generated either enzymatically (using horseradish peroxidase-H2O2 or tyrosinase-O2) or by autoxidation
Patrick Mazellier et al.
Chemosphere, 73(8), 1216-1223 (2008-09-03)
The photochemical transformation of natural estrogenic steroid 17beta-estradiol (E2) and the synthetic oral contraceptive 17alpha-ethinylestradiol (EE2) has been studied in dilute non buffered aqueous solution (pH 5.5-6.0) upon monochromatic (254 nm) and polychromatic (lambda>290 nm) irradiation. Upon irradiation at 254
N M Howarth et al.
Steroids, 62(4), 346-350 (1997-04-01)
In our continuing quest to design efficient inhibitors of estrone sulfatase activity and to assess the recognition of estrone sulfate surrogates by estrone sulfatase, we synthesized and evaluated several sulfonate derivatives of 5,6,7,8-tetrahydronaphth-2-ol and estrone. 5,6,7,8-Tetrahydronaphth-2-methanesulfonate (11), and 5,6,7,8-tetrahydronaphth-2-(p-toluene)sulfonate (12)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.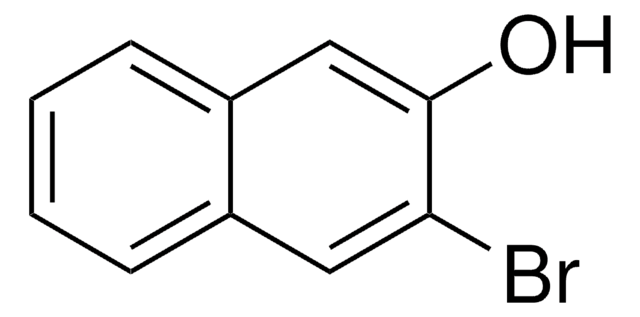

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
