추천 제품
일반 설명
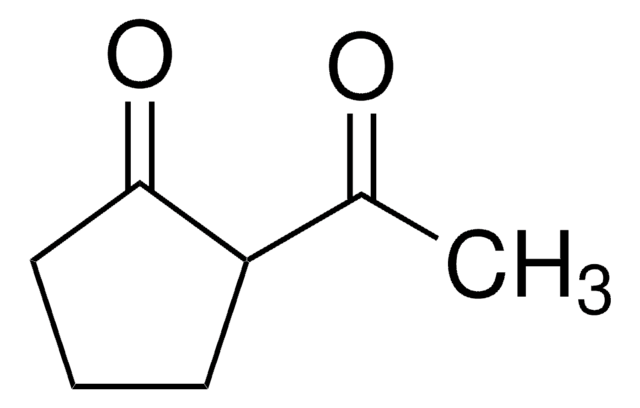
The keto-enol tautomerism of 2-acetylcyclohexanone (ACHE) in water was studied.
애플리케이션
2-Acetylcyclohexanone was used in the synthesis of anilinoethanolamines.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
174.2 °F - closed cup
Flash Point (°C)
79 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Emilia Iglesias
The Journal of organic chemistry, 68(7), 2680-2688 (2003-03-29)
The keto-enol tautomerism of 2-acetylcyclohexanone (ACHE) was studied in water under different experimental conditions. By contrast with other previously studied beta-diketones, the keto-enol interconversion in the ACHE system is a slow process. Under equilibrium conditions, the analysis of the absorbance
Cédric Bouteiller et al.
Organic & biomolecular chemistry, 8(5), 1111-1120 (2010-02-19)
An operationally simple and concise synthesis of anilinoethanolamines, as NMDA NR2B receptor antagonist ifenprodil analogues, was developed via a copper-catalyzed amination of the corresponding bromoarene. Coupling was achieved with linear primary alkylamines, alpha,omega-diamines, hexanolamine and benzophenone imine, as well as
Emilia Iglesias
The Journal of organic chemistry, 68(7), 2689-2697 (2003-03-29)
The kinetic study of the nitrosation of the enol of 2-acetylcyclohexanone (ACHE) has been performed in aqueous acid media in the absence and presence of alpha- and beta-cyclodextrin. The reaction is first-order with respect to both reactants concentration: [nitrite] and
An alternative to the classical α-arylation: the transfer of an intact 2-iodoaryl from ArI(O₂CCF₃)₂.
Zhiyu Jia et al.
Angewandte Chemie (International ed. in English), 53(42), 11298-11301 (2014-09-10)
The α-arylation of carbonyl compounds is generally accomplished under basic conditions, both under metal catalysis and via aryl transfer from the diaryl λ(3)-iodanes. Here, we describe an alternative metal-free α-arylation using ArI(O2CCF3)2 as the source of a 2-iodoaryl group. The
Masaharu Fujita et al.
Journal of applied toxicology : JAT, 39(2), 191-208 (2018-09-18)
The amino acid derivative reactivity assay (ADRA) is an in chemico alternative to animal testing for skin sensitization that solves certain problems found in the use of the direct peptide reactivity assay (DPRA). During a recent validation study conducted at
문서
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)